Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
- use only with enclosed dosing cup
| adults and children 6 years and over | 2 teaspoonfuls (tsp) daily; do not take more than 2 teaspoonfuls (tsp) in 24 hours |
| children 2 to under 6 years of age | 1 teaspoonful (tsp) daily; do not take more than 1 teaspoonful (tsp) in 24 hours |
| children under 2 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
- each teaspoonful contains: sodium 5 mg
- do not use if bottle wrap imprinted with "SEALED FOR SAFETY" is broken or missing.
- store between 20° and 25°C (68° and 77°F)
- see bottom panel for lot number and expiration date
Inactive ingredients
bubble gum flavor, butylated hydroxyanisole, glycerin, maltitol solution, noncrystallizing sorbitol solution, phosphoric acid, polyethylene glycol, propylene glycol, purified water, sodium benzoate, sodium phosphate monobasic dihydrate, sucralose powder
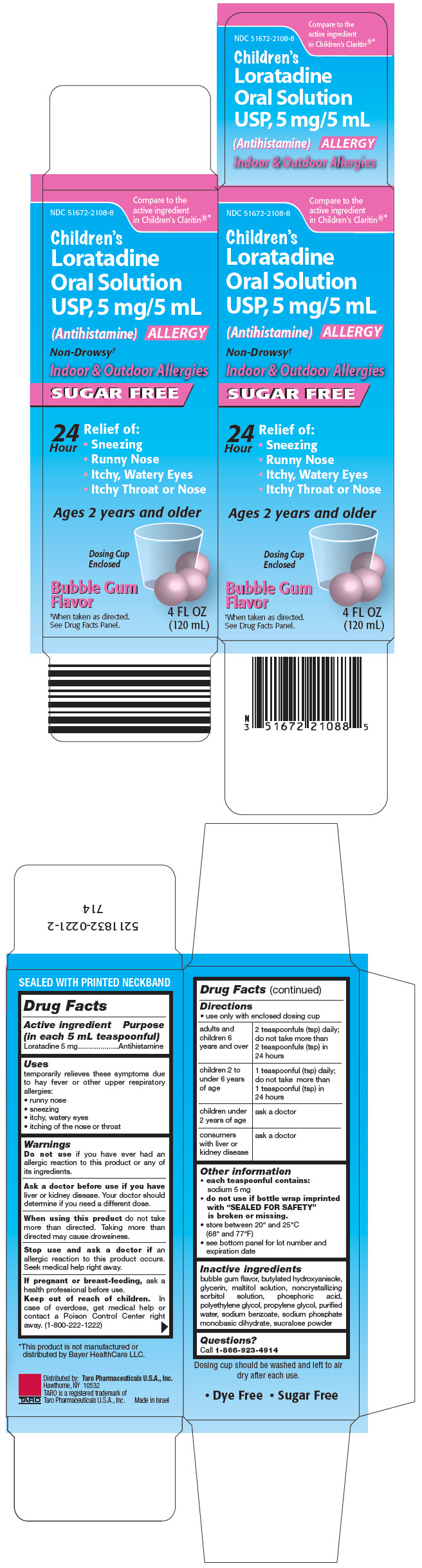
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Carton
Compare to the
active ingredient
in Children's Claritin®*
NDC 51672-2108-8
Children's
Loratadine
Oral Solution
USP, 5 mg/5 mL
(Antihistamine) ALLERGY
Non-Drowsy†
Indoor & Outdoor Allergies
SUGAR FREE
24
Hour
Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
Ages 2 years and older
Dosing Cup
Enclosed
Bubble Gum
Flavor
†When taken as directed.
See Drug Facts Panel.
4 FL OZ
(120 mL)