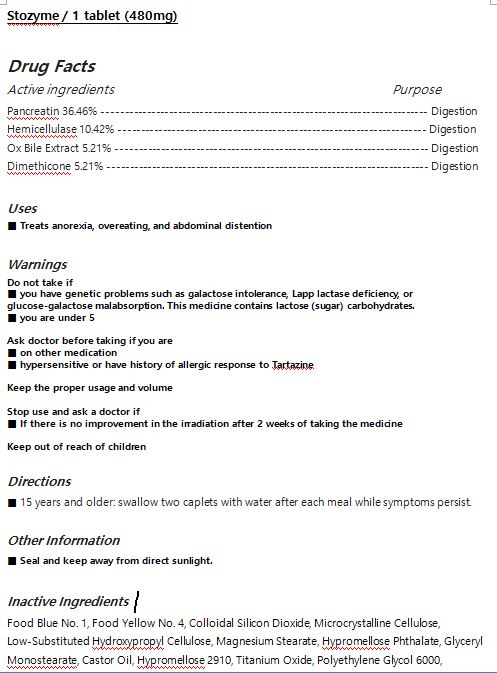
INACTIVE INGREDIENT
Food Blue No. 1, Food Yellow No. 4, Colloidal Silicon Dioxide, Microcrystalline Cellulose, Low-Substituted Hydroxypropyl Cellulose, Magnesium Stearate, Hypromellose Phthalate, Glyceryl Monostearate, Castor Oil, Hypromellose 2910, Titanium Oxide, Polyethylene Glycol 6000,
WARNING
Do not take if
-you have genetic problems such as galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption. This medicine contains lactose (sugar) carbohydrates.
-you are under 5
Ask doctor before taking if
- you are on other medication
- you are hypersensitive or have history of allergic response to Tartazine
Keep the proper usage and volume
Stop use and ask a doctor if
- If there is no improvement in the irradiation after 2 weeks of taking the medicine