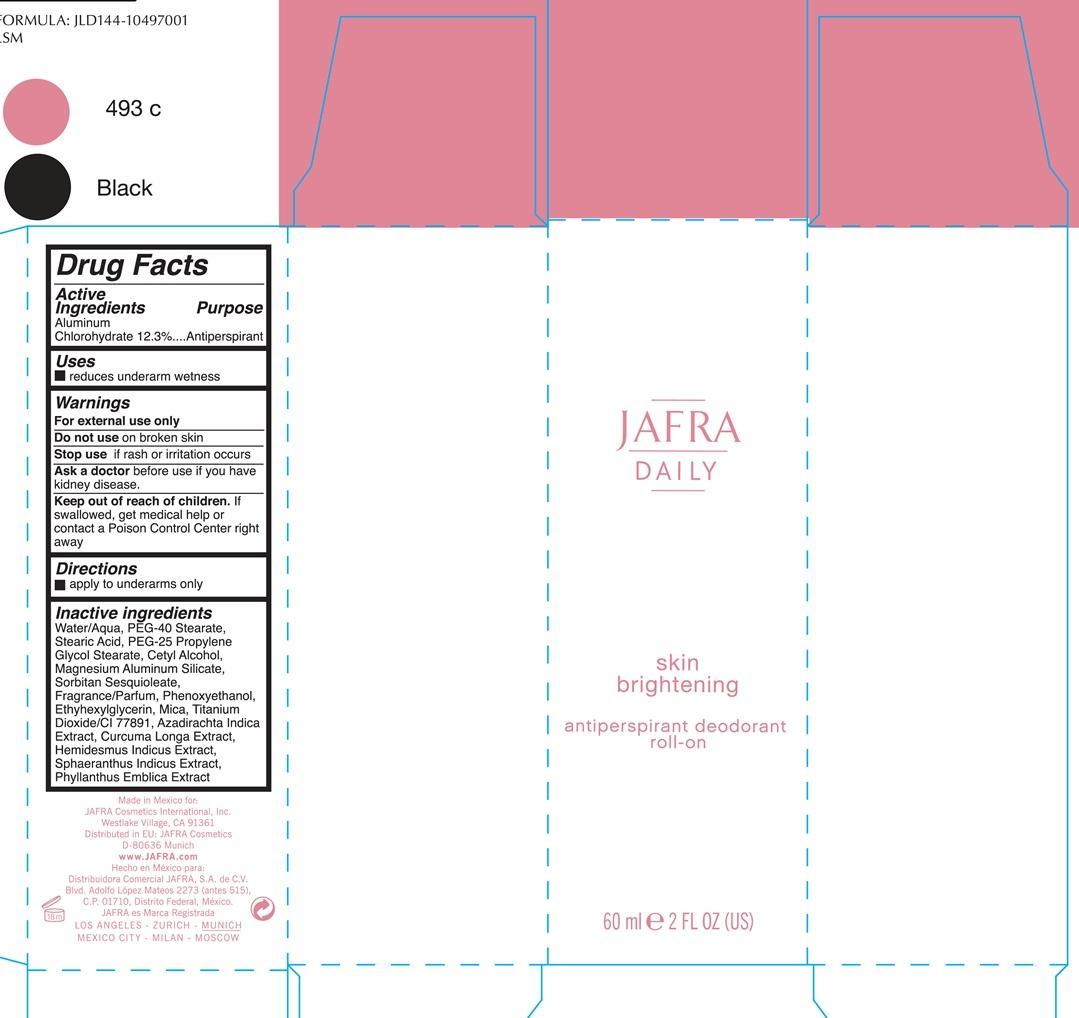
Active ingredients Purpose
Aluminum Chlorohydrate 12.3% Antiperspirant
Uses
reduces underarm wetness
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Stop use if rash or irritation occurs
Warnings
For external use only
Do not use on broken skin
Ask a doctor before before use if you have kidney disease
Directions
Apply to underarms only
Water/Aqua, PEG-40 Stearate, PEG-25 Propylene Glycol Stearate, Cetyl Alcohol, Magnesium Aluminum Silicate, Sorbitan Sesquioleate, Fragrance/parfum, Phenoxyethanol, Ehtylhexylhexyl Glycerin, Mica, Titanium Dioxide/CI 77891, Azadirachta Indica Extract, Curcuma Longa, Hemisdesmus Indicus, Sphaeranthus Indicus Extract, Phyllanthus Emblica Extract