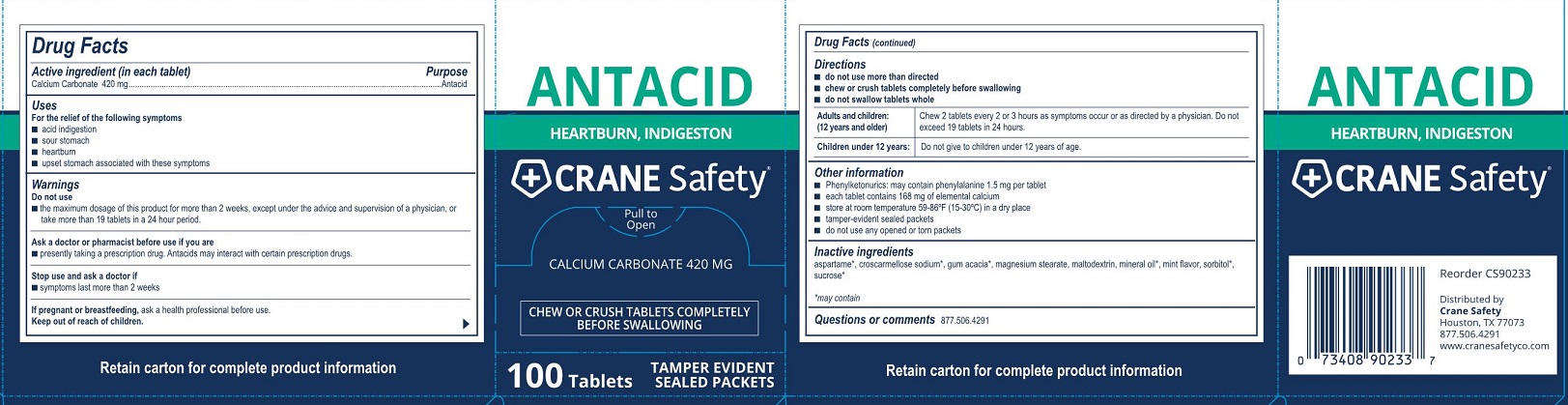
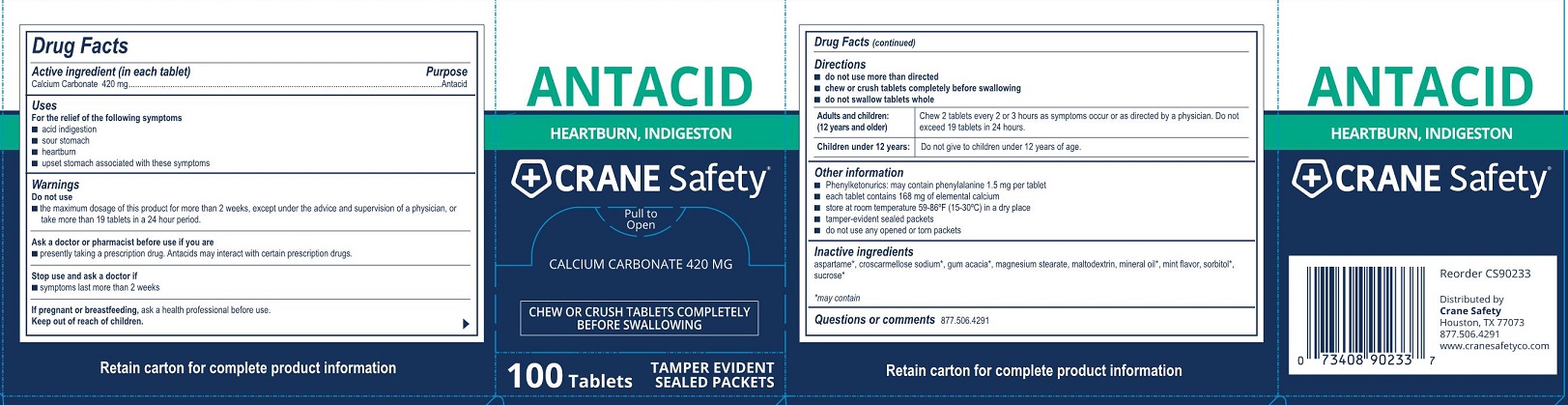
Do not use
- the maximum dosage of this product for more than 2 weeks except under the advice and supervision of a doctor
Ask a doctor or pharmacists before use if you are
- are taking a prescription drug. Antacids may interact with certain prescription drugs.
- have kidney disease
When usung this product
- do not take more than 19 tablets in a 24 hour period. If symptoms persist for more than 2 weeks, stop using this product and see a doctor.
Directions
Adults and children: (12 years and older) Chew 2 tablets every 2 - 3 hours as symptoms occur, repeat hourly if needed. Do not exceed19 tablets in 24 hours.
Children under 12 years: Ask a doctor
Other information
- calcium content per tablet: 168 mg
- phenylketonurics: each tablet may contain 1.5 mg phenylalanine
- store at room temperature 59-86°F (15-30°C) in a dry place
- tamper-evident sealed packets
- do not use any opened or torn packets
Inactive ingredients aspartame*, croscarmellose sodium*, gum acacia*, magnesium stearate, maltodextrin, mineral oil*, mint flavor, sorbitol*, sucrose*
*may contain