APLICARE POVIDONE-IODINE SCRUB - povidone-iodine solution
Aplicare, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Povidone-iodine Scrub Swabstick Packet
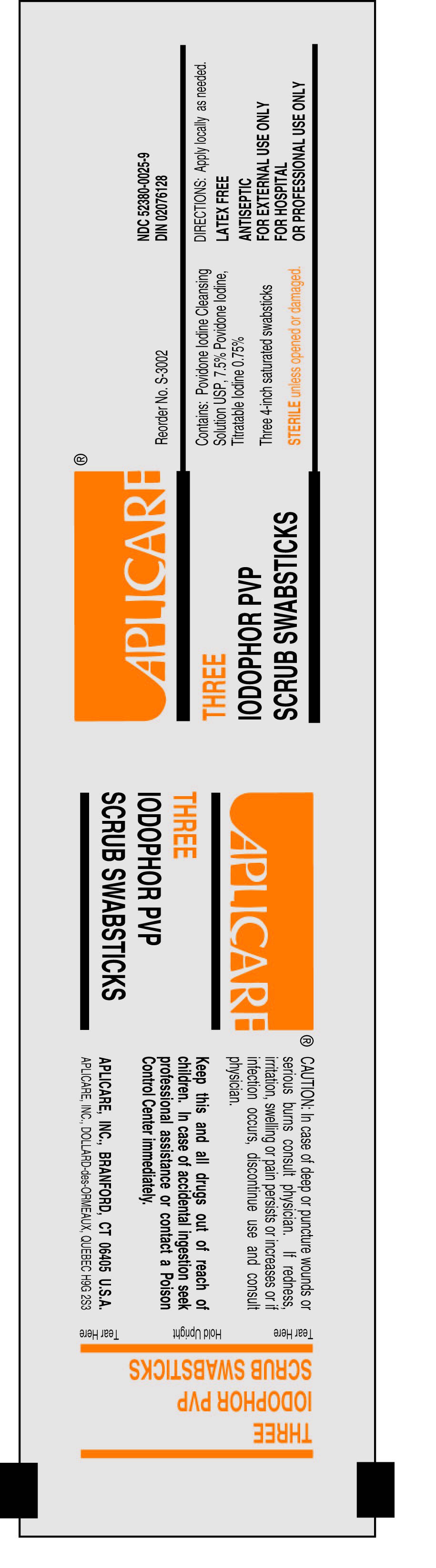
Stop use and ask a doctor if
-
redness,
irritation, swelling or pain persists or increases
-
infection
occurs
-
injuries
are deep or puncture wounds or serious burns
Do not use if allergic to iodine
Keep out of reach of
children. In case of accidental
ingestion, seek professional assistance or consult a poison control center
immediately.
Questions or comments?
1 800
760-3236 (Mon to Fri 8:30 AM - 5:00 PM EST)
Aplicare, Inc.