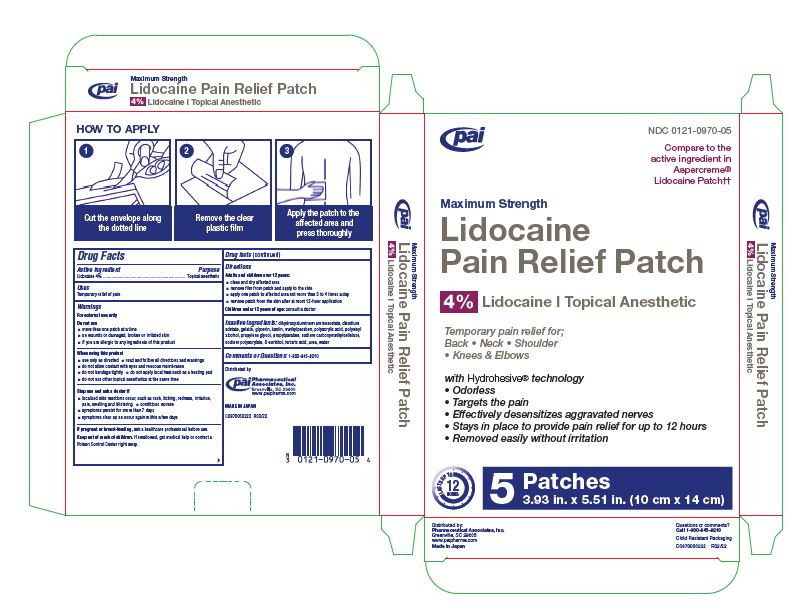
Warnings
Do not use
- more than one patch at a time
- on wounds or damaged, broken or irritated skin
- if you are allergic to any ingredients of this product
When using this product
- use only as directed • read and follow all directions and warnings
- do not allow contact with eyes and mucous membranes
- do not bandage tightly • do not apply local heat such as a heating pad
- do not use other topical anesthetics at the same time
Directions
Adults and children over 12 years:
- clean and dry affected area
- remove film from patch and apply to the skin
- apply one patch to affected area not more than 3 to 4 times a day
- remove patch from the skin after at most 12-hour application
Children under 12 years of age: consult a doctor
Inactive ingredients
dihydroxyaluminum aminoacetate, disodium edetate, gelatin, glycerin, kaolin, methylparaben, polyacrylic acid, polyvinyl
alcohol, propylene glycol, propylparaben, sodium carboxymethylcellulose, sodium polyacrylate, D-sorbitol, tartaric acid, urea, water