Active Ingredient
Salicylic Acid, 2%
Use:
Controls scalp itching and flaking due to dandruff
Warnings
For external use only
When using this product
avoid contact with eyes. If contact, rinse eyes thoroughly with water.
Stop use and consult a doctor if
condition worsens or does not improve after regular use of this product as directed.
Keep out of reach of children.
If swallowed, get medical help or call a Poison Control Center at once.
Directions
Spray to apply to the affected area 1-4 times daily, or as directed by a doctor
Inactive Ingredients
Water, Glycerin, Sodium Hydroxide, PEG-40 Hydrogenate Castor Oil, PEG-12 Dimethicone, Propylene Glycol, Hydrolyzed Collagen, Diazolidinyl Urea, Disodium EDT, Methylparaben, Propylparaben, Ethylhexylglycerin, PEG-12 Alyl Ether, PEG-12, Butylene Glycol, Phenoxyethanol, Fragrance
Package Labeling:
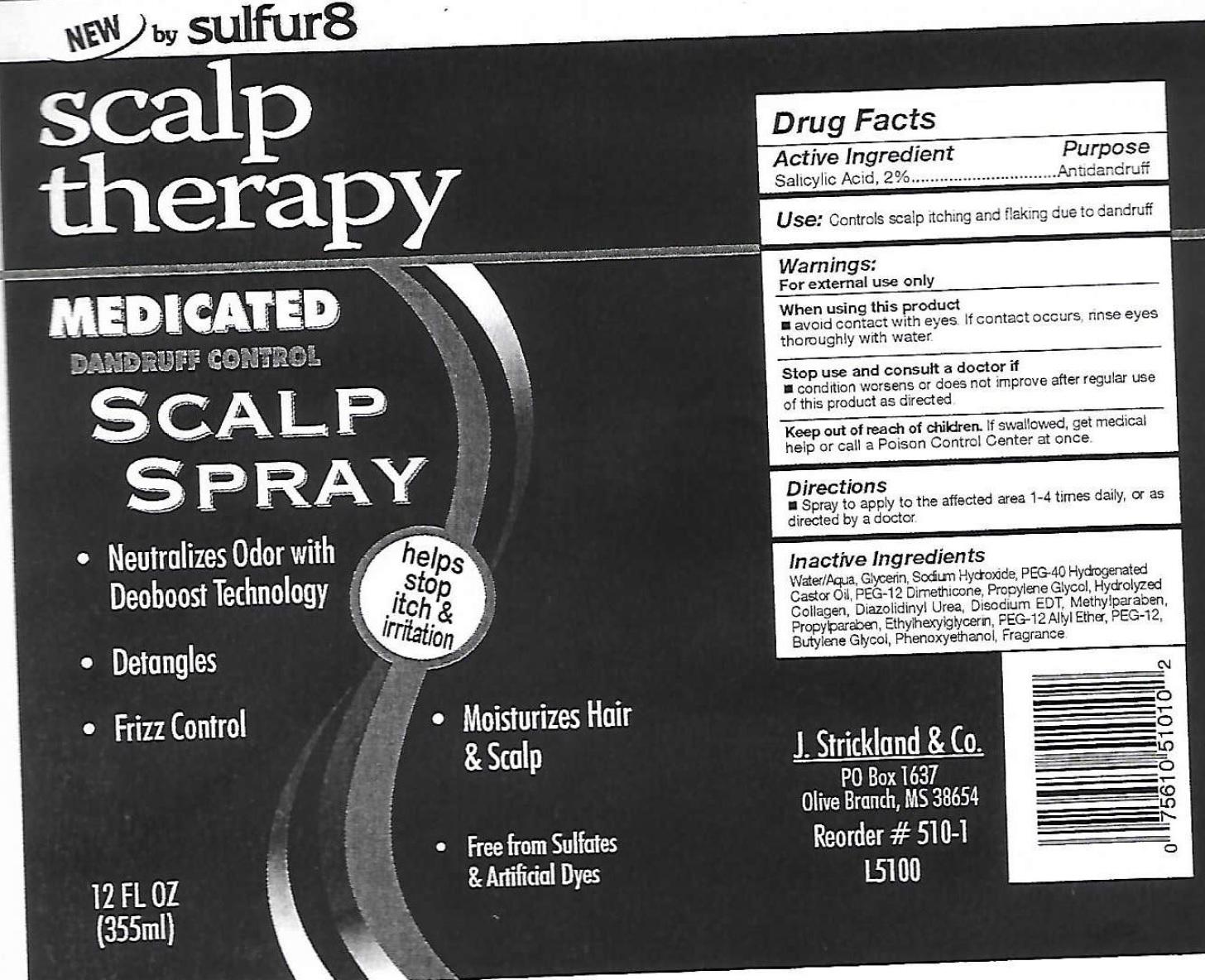
J. Strickland and Co.