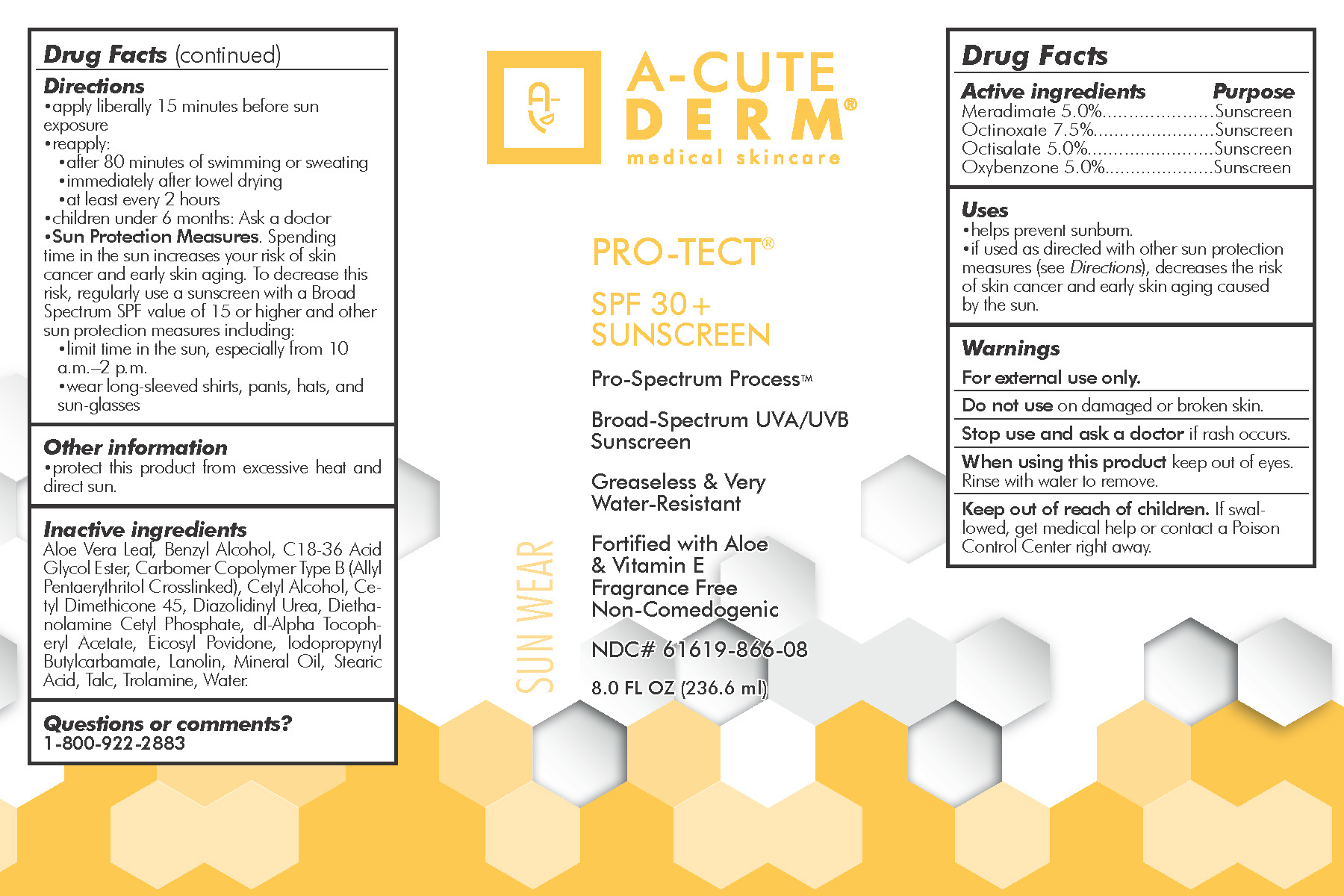
Uses
•helps prevent sunburn.
•if used as directed with other sun protection
measures (see Directions), decreases the risk
of skin cancer and early skin aging caused
by the sun.
Warnings
For external use only.
Do not use on damaged or broken skin.
Stop use and ask a doctor if rash occurs.
When using this product keep out of eyes.
Rinse with water to remove.
Keep out of reach of children. If swallowed,
get medical help or contact a Poison
Control Center right away.
Directions
•apply liberally 15 minutes before sun
exposure
•reapply:
•after 80 minutes of swimming or sweating
•immediately after towel drying
•at least every 2 hours
•children under 6 months: Ask a doctor
•
Sun Protection Measures. Spending
time in the sun increases your risk of skin
cancer and early skin aging. To decrease this
risk, regularly use a sunscreen with a Broad
Spectrum SPF value of 15 or higher and other
sun protection measures including:
•limit time in the sun, especially from 10
a.m.–2 p.m.
•wear long-sleeved shirts, pants, hats, and
sun-glasses
Other Information
• Daily usage of Pro-Tect® SPF 30+ may help reduce the chance of premature aging of the skin due to overexposure to the sun. • This product provides more than 30 times your natural protection against sunburn. • Very Water Resistant UVA & UVB Broad-Spectrum Protection. • This product may stain light colored fabrics if washed with bleach. Wash clothing without bleach to remove safely.
Inactive Ingredients
Aloe Vera Leaf, Benzyl Alcohol, C18-36 Acid Glycol Ester, Carbomer Copolymer Type B (Allyl Pentaerythritol Crosslinked), Cetyl Alcohol, Cetyl Dimethicone 45, Diazolidinyl Urea, Diethanolamine Cetyl Phosphate, dl-Alpha Tocopheryl Acetate, Eicosyl Povidone, Iodopropynyl Butylcarbamate, Lanolin, Mineral Oil, Stearic Acid, Talc, Trolamine, Water.