Use:
For preparation prior to surgery. Helps to reduce bacteria that can potentially cause skin infection.
Do not apply to persons allergic to iodine. Do not use in the eyes.
Directions:
Clean the area. Apply product to the operative site prior to surgery using pad to prep desired area.
Other Information:
1% titratable iodine, for hospital or professional use only. Store at ambient temperatures.
Inactive Ingredients:
Citric Acid, Glycerin, Sodium Hydroxide, Potassium Iodide, Alkyl Glucoside, Nonoxynol-10, Hydroxyethyl Cellulose and Purified Water.
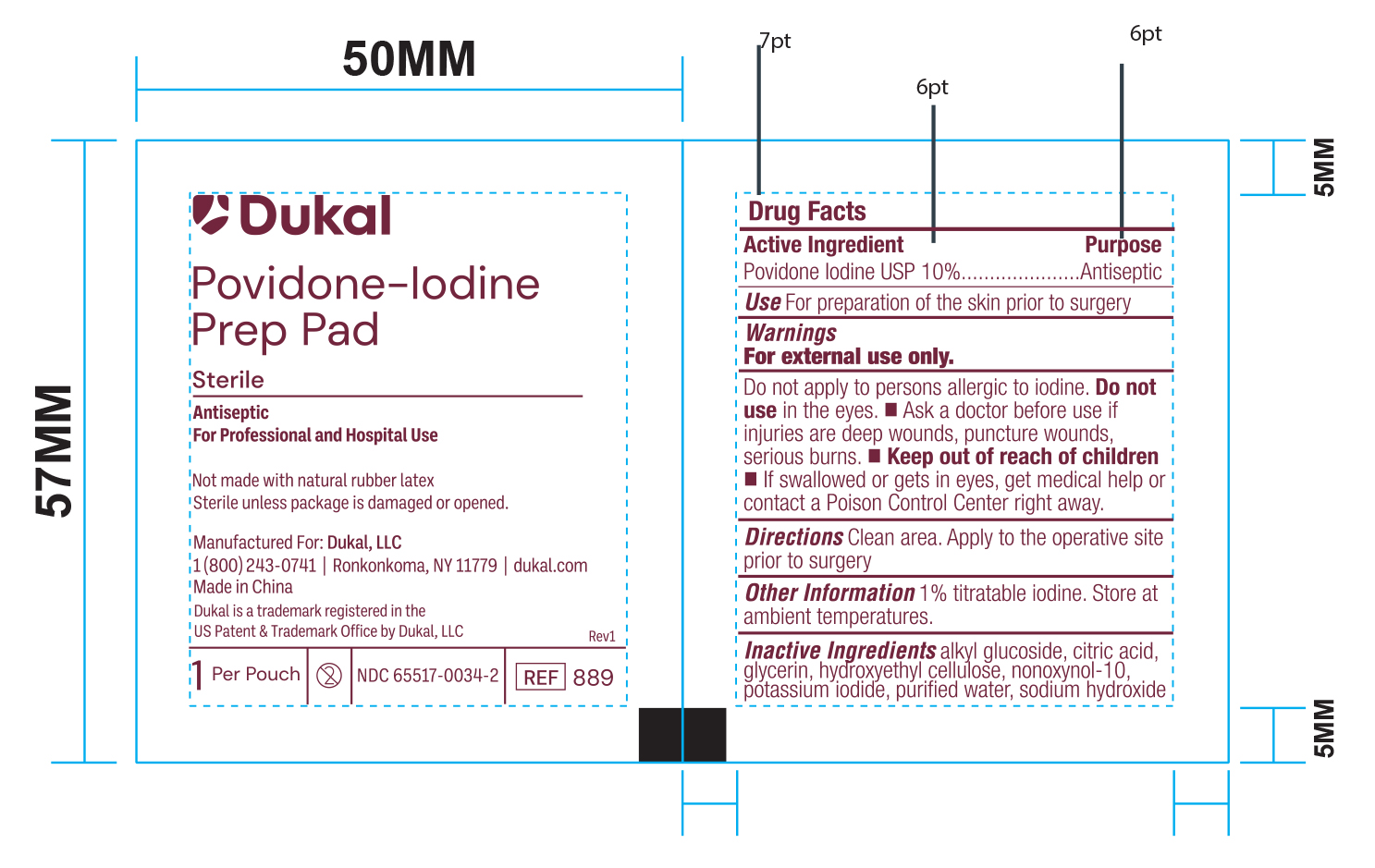
Principal Display Panel - PVP-I Prep Pads Foil Pouch Label
Dukal
Povidone-Iodine Prep Pad
Sterile
Antiseptic
For Professional and Hospital Use
Not made with natura rubber latex
Sterile unless package is damaged or opened.
Manufactured For: Dukal, LLC
1(800)243-0741 / Ronkonkoma, NY 11779 / dukal.com
Made in China
Dukal is a trademark registered in the
US Patent & Trademark Office by Dukal, LLC
1 Per Pouch - 2 - NDC 6551-0034-1 - REF 882
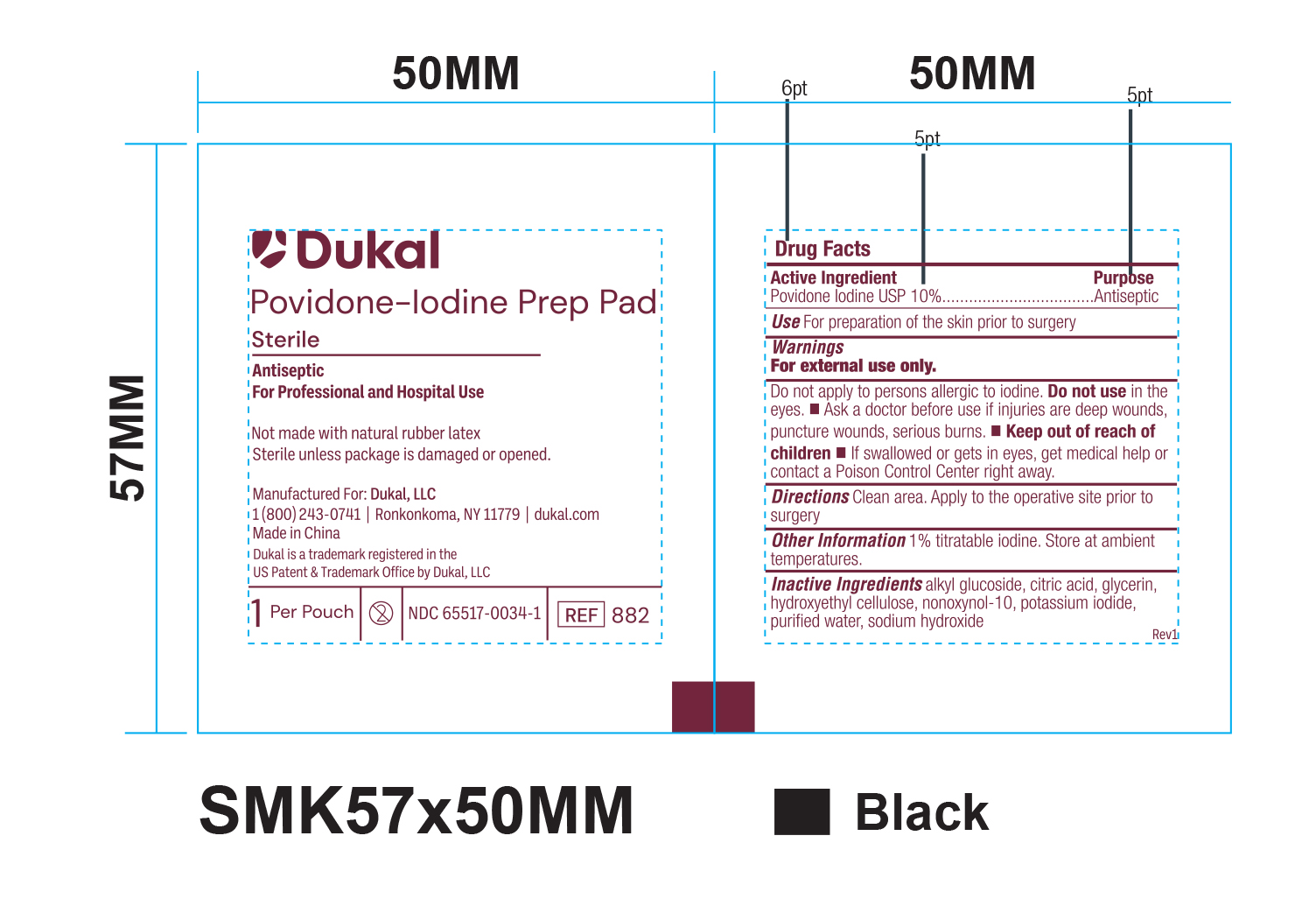
Principal Display Panel - PVP-I Prep Pads Paper Pouch Label
Dukal
Povidone-Iodine Prep Pad
Sterile
Antiseptic
For Professional and Hospital Use
Not made with natura rubber latex
Sterile unless package is damaged or opened.
Manufactured For: Dukal, LLC
1(800)243-0741 / Ronkonkoma, NY 11779 / dukal.com
Made in China
Dukal is a trademark registered in the
US Patent & Trademark Office by Dukal, LLC Rev1
1 Per Pouch - 2 - NDC 6551-0034-2 - REF 889