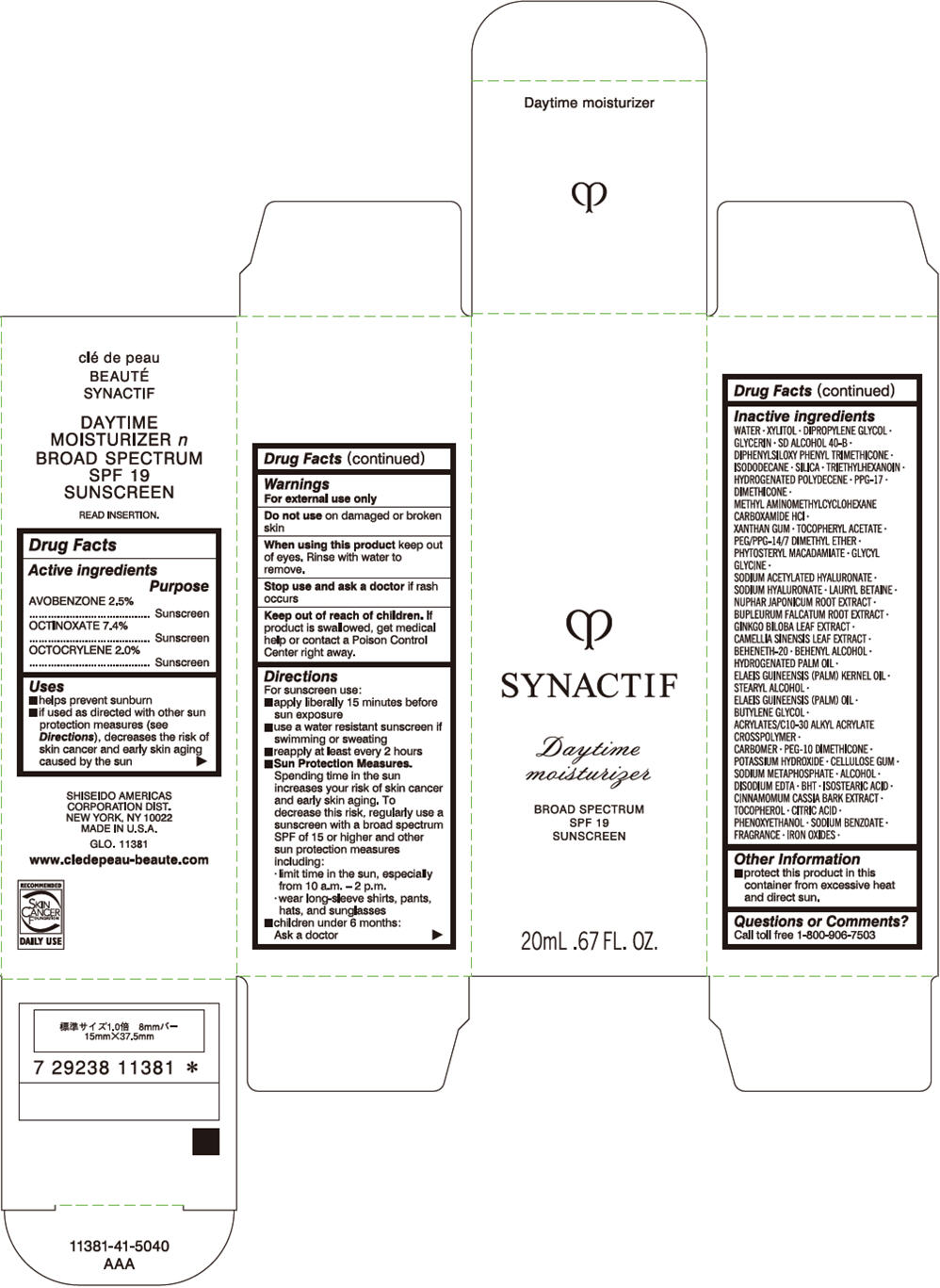
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
WATER∙XYLITOL∙DIPROPYLENE GLYCOL∙GLYCERIN∙SD ALCOHOL 40-B∙DIPHENYLSILOXY PHENYL TRIMETHICONE∙ISODODECANE∙SILICA∙TRIETHYLHEXANOIN∙HYDROGENATED POLYDECENE∙PPG-17∙DIMETHICONE∙METHYL AMINOMETHYLCYCLOHEXANE CARBOXAMIDE HCl∙XANTHAN GUM∙TOCOPHERYL ACETATE∙PEG/PPG-14/7 DIMETHYL ETHER∙PHYTOSTERYL MACADAMIATE∙GLYCYL GLYCINE∙SODIUM ACETYLATED HYALURONATE∙SODIUM HYALURONATE∙LAURYL BETAINE∙NUPHAR JAPONICUM ROOT EXTRACT∙BUPLEURUM FALCATUM ROOT EXTRACT∙GINKGO BILOBA LEAF EXTRACT∙CAMELLIA SINENSIS LEAF EXTRACT∙BEHENETH-20∙BEHENYL ALCOHOL∙HYDROGENATED PALM OIL∙ELAEIS GUINEENSIS (PALM) KERNEL OIL∙STEARYL ALCOHOL∙ELAEIS GUINEENSIS (PALM) OIL∙BUTYLENE GLYCOL∙ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER∙CARBOMER∙PEG-10 DIMETHICONE∙POTASSIUM HYDROXIDE∙CELLULOSE GUM∙SODIUM METAPHOSPHATE∙ALCOHOL∙DISODIUM EDTA∙BHT∙ISOSTEARIC ACID∙CINNAMOMUM CASSIA BARK EXTRACT∙TOCOPHEROL∙CITRIC ACID∙PHENOXYETHANOL∙SODIUM BENZOATE∙FRAGRANCE∙IRON OXIDES∙