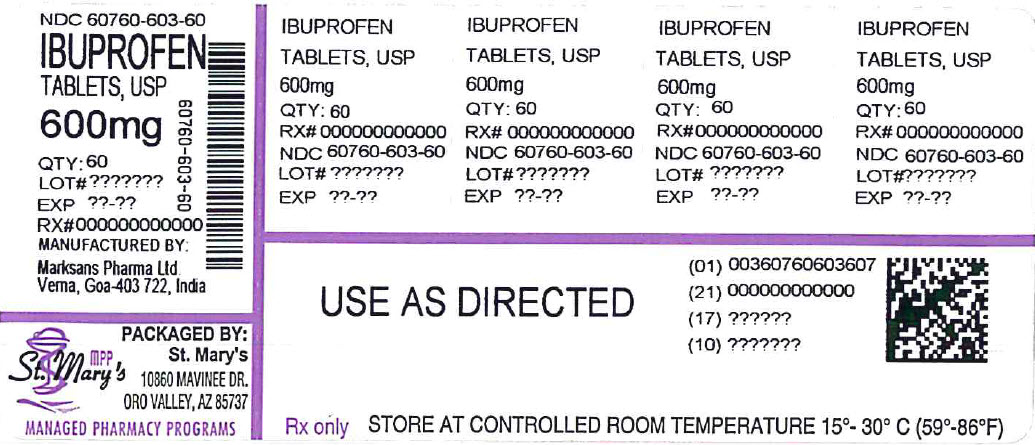
HOW SUPPLIED
600mg (white to off white, capsule shaped, biconvex, film coated tablets debossed with '122' on one side and plain on the other side)
NDC
60760-603-20 BOTTLES OF 20
60760-603-28 BOTTLES OF 28
60760-603-30 BOTTLES OF 30
60760-603-40 BOTTLES OF 40
60760-603-60 BOTTLES OF 60
60760-603-90 BOTTLES OF 90