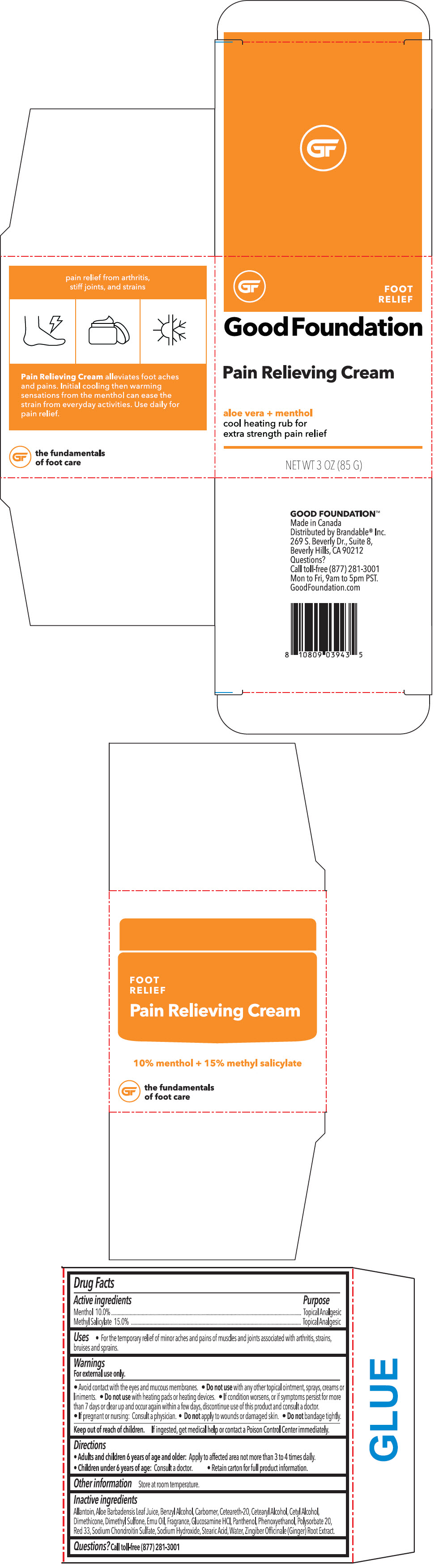
PAIN RELIEVING FOOT- menthyl salicylate and menthol, unspecified form cream
Brandable
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients | Purpose |
| Menthol 10.0% | Topical Analgesic |
| Methyl Salicylate 15.0% | Topical Analgesic |
Uses
- For the temporary relief of minor aches and pains of muscles and joints associated with arthritis, strains, bruises and sprains.
Warnings
For external use only.
- Avoid contact with the eyes and mucous membranes.
-
Do not use with any other topical ointment, sprays, creams or liniments.
-
Do not use with heating pads or heating devices.
- If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
- If pregnant or nursing: Consult a physician.
-
Do not apply to wounds or damaged skin.
-
Do not bandage tightly.
Keep out of reach of children. If ingested, get medical help or contact a Poison Control Center immediately.
Directions
-
Adults and children 6 years of age and older: Apply to affected area not more than 3 to 4 times daily.
-
Children under 6 years of age: Consult a doctor.
- Retain carton for full product information.
Other information
Store at room temperature.
Inactive ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Benzyl Alcohol, Carbomer, Ceteareth-20, Cetearyl Alcohol, Cetyl Alcohol, Dimethicone, Dimethyl Sulfone, Emu Oil, Fragrance, Glucosamine HCl, Panthenol, Phenoxyethanol, Polysorbate 20, Red 33, Sodium Chondroitin Sulfate, Sodium Hydroxide, Stearic Acid, Water, Zingiber Officinale (Ginger) Root Extract.
Questions?
Call toll-free (877) 281-3001
Distributed by Brandable® Inc.
269 S. Beverly Dr., Suite 8,
Beverly Hills, CA 90212
PRINCIPAL DISPLAY PANEL - 85 G Container Box
FOOT
RELIEF
Good Foundation
Pain Relieving Cream
aloe vera + menthol
cool heating rub for
extra strength pain relief
NET WT 3 OZ (85 G)