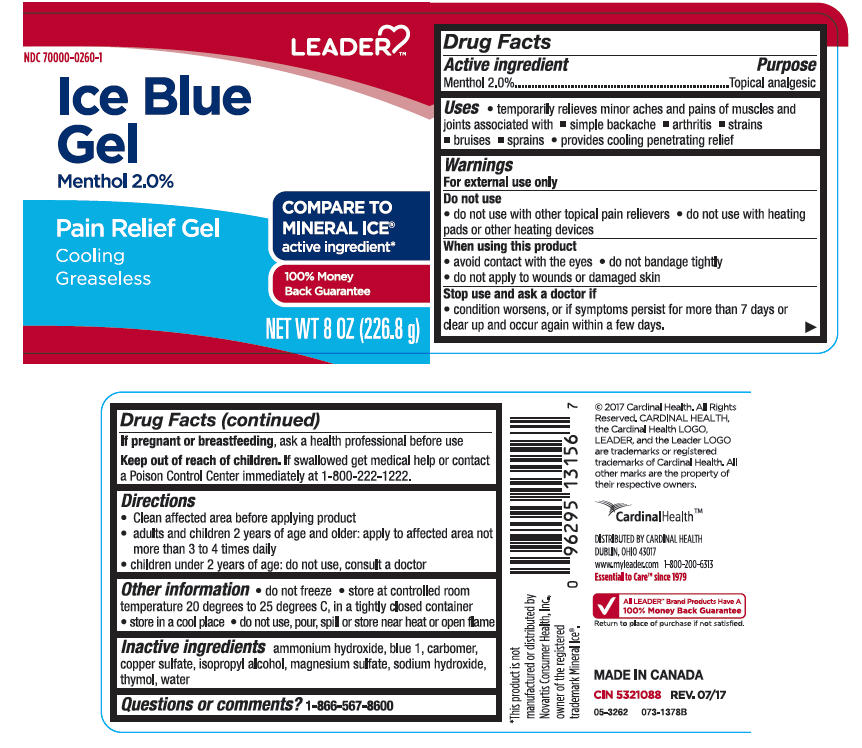
Uses
- ♦
- temporarily relieves minor aches and pains of muscles and joints associated with
- ♦
- simple backache
- ♦
- arthritis
- ♦
- strains
- ♦
- bruises
- ♦
- sprains
- ♦
- provides cooling penetrating relief
Warnings
For external use only
Do not use
- ♦
- do not use with other topical pain relievers
- ♦
- do not use with heating pads or other heating devices
When using this product
- ♦
- avoid contact with the eyes
- ♦
- do not bandage tightly
- ♦
- do not apply to wounds or damaged skin
Directions
- ♦
- Clean affected area before applying product
- ♦
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- ♦
- children under 2 years of age: do not use, consult a doctor
Other information
- ♦
- do not freeze
- ♦
- store at controlled room temperature 20 degrees to 25 degrees C, in a tightly closed container
- ♦
- store in a cool place
- ♦
- do not use, pour, spill or store near heat or open flame