DESCRIPTION
Cyproheptadine Hydrochloride 2 mg Alcohol 5%, citric acid, D&C Yellow #10, flavors, purified water, sodium citrate, sorbic acid (0.1% as preservative) and sucrose syrup.
Each 5 mL (one teaspoonful) contains:
Inactive Ingredients:
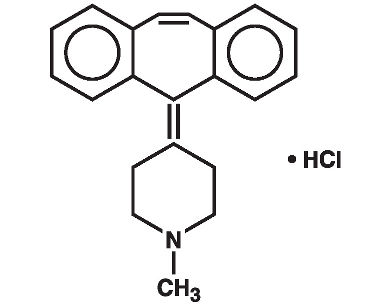
Cyproheptadine HCI is an antihistaminic and antiserotonergic agent. Cyproheptadine hydrochloride is a white to slightly yellowish, crystalline solid, with a molecular weight of 350.89, which is slightly soluble in water, freely soluble in methanol, sparingly soluble in ethanol, soluble in chloroform and practically insoluble in ether. It is the sesquihydrate of 4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine hydrochloride. The molecular formula of the anhydrous salt is C H N•HCl and the structural formula of the anhydrous salt is: 2121
CLINICAL PHARMACOLOGY
Cyproheptadine is a serotonin and histamine antagonist with anticholinergic and sedative effects. Antiserotonin and antihistamine drugs appear to compete with serotonin and histamine, respectively, for receptor sites.
After a single 4 mg oral dose of C-labeled cyproheptadine HCI in normal subjects, given as tablets or syrup, 2-20% of the radioactivity was excreted in the stools. Only about 34% of the stool radioactivity was unchanged drug, corresponding to less than 5.7% of the dose. At least 40% of the administered radioactivity was excreted in the urine. No detectable amounts of unchanged drug were present in the urine of patients on chronic 12-20 mg daily doses of cyproheptadine syrup. The principal metabolite found in human urine has been identified as a quaternary ammonium glucuronide conjugate of cyproheptadine. Elimination is diminished in renal insufficiency. Pharmacokinetics and Metabolism:14
INDICATIONS AND USAGE
Perennial and seasonal allergic rhinitis Vasomotor rhinitis Allergic conjunctivitis due to inhalant allergens and foods Mild, uncomplicated allergic skin manifestations of urticaria and angioedema Amelioration of allergic reactions to blood or plasma Cold urticaria Dermatographism
As therapy for anaphylactic reactions to epinephrine and other standard measures after the acute manifestations have been controlled.
adjunctive
CONTRAINDICATIONS
This drug should be used in newborn or premature infants. Newborn or Premature Infants:not
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers. Nursing Mothers:
Hypersensitivity to cyproheptadine and other drugs of similar chemical structure Monoamine oxidase inhibitor therapy (see ) Angle-closure glaucoma Stenosing peptic ulcer Symptomatic prostatic hypertrophy Bladder neck obstruction Pyloroduodenal obstruction Elderly, debilitated patients
Other Conditions:
Drug Interactions
WARNINGS
Overdosage of antihistamines, particularly in infants and children, may produce hallucinations, central nervous system depression, convulsions and death. Children:
Antihistamines may diminish mental alertness; conversely, particularly in the young child, they may occasionally produce excitation.
Antihistamines may have additive effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, tranquilizers, antianxiety agents. CNS Depressants:
Patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a car or operating machinery. Activities Requiring Mental Alertness:
Antihistamines are more likely to cause dizziness, sedation and hypotension in elderly patients.
PRECAUTIONS
Cyproheptadine has an atropine-like action and, therefore, should be used with caution in patients with:
General:- History of bronchial asthma
- Increased intraocular pressure
- Hyperthyroidism
- Cardiovascular disease
- Hypertension
Antihistamines may diminish mental alertness; conversely, particularly in the young child, they may occasionally produce excitation. Patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a car or operating machinery. Information for Patients:
MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines. Antihistamines may have additive effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, tranquilizers, antianxiety agents. Drug Interactions:
Long-term carcinogenic studies have not been done with cyproheptadine. Cyproheptadine had no effect on fertility in a two-litter study in rats or a two-generation study in mice at about 10 times the human dose. Cyproheptadine did not produce chromosome damage in human lymphocytes or fibroblasts ; high doses (10 M) were cytotoxic. Cyproheptadine did not have any mutagenic effect in the Ames microbial mutagen test; concentrations of above 500 mcg/plate inhibited bacterial growth. Carcinogenesis, Mutagenesis, Impairment of Fertility:in vitro-4
Pregnancy Category B. Reproduction studies have been performed in rabbits, mice and rats at oral or subcutaneous doses up to 32 times the maximum recommended human oral dose and have revealed no evidence of impaired fertility or harm to the fetus due to cyproheptadine. Cyproheptadine has been shown to be fetotoxic in rats when given by intraperitoneal injection in doses four times the maximum recommended human oral dose. Two studies in pregnant women, however, have not shown that cyproheptadine increases the risk of abnormalities when administered during the first, second and third trimesters of pregnancy. No teratogenic effects were observed in any of the newborns. Nevertheless, because the studies in humans cannot rule out the possibility of harm, cyproheptadine should be used during pregnancy only if clearly needed. Pregnancy:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from cyproheptadine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see ). Nursing Mothers:CONTRAINDICATIONS
Safety and effectiveness in pediatric patients below the age of two years have not been established. (See , and .) Pediatric Use:CONTRAINDICATIONS, Newborn or Premature InfantsWARNINGS, Children
ADVERSE REACTIONS
Adverse reactions which have been reported with the use of antihistamines are as follows:
Sedation and sleepiness (often transient), dizziness, disturbed coordination, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, paresthesias, neuritis, convulsions, euphoria, hallucinations, hysteria, faintness. Central Nervous System:
Allergic manifestation of rash and edema, excessive perspiration, urticaria, photosensitivity. Integumentary:
Acute labyrinthitis, blurred vision, diplopia, vertigo, tinnitus. Special Senses:
Hypotension, palpitation, tachycardia, extrasystoles, anaphylactic shock. Cardiovascular:
Hemolytic anemia, leukopenia, agranulocytosis, thrombocytopenia. Hematologic:
Dryness of mouth, epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation, jaundice. Digestive System:
Urinary frequency, difficult urination, urinary retention, early menses. Genitourinary:
Dryness of nose and throat, thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness. Respiratory:
Fatigue, chills, headache, increased appetite/weight gain. Miscellaneous:
OVERDOSAGE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation especially in children. Also, atropine-like signs and symptoms (dry mouth; fixed, dilated pupils; flushing, etc.) as well as gastrointestinal symptoms may occur.
the patient should be induced to vomit with syrup of ipecac. If vomiting has not occurred spontaneously,
perform gastric lavage followed by activated charcoal. Isotonic or 1/2 isotonic saline is the lavage of choice. Precautions against aspiration must be taken especially in infants and children. When life-threatening CNS signs and symptoms are present, intravenous physostigmine salicylate may be considered. Dosage and frequency of administration are dependent on age, clinical response and recurrence after response. (See package circulars for physostigmine products.) If the patient is unable to vomit,
as milk of magnesia, by osmosis draw water into the bowel and, therefore, are valuable for their action in rapid dilution of bowel content. Saline cathartics,
should be used. Vasopressors may be used to treat hypotension. Stimulantsnot
The oral LD of cyproheptadine is 123 mg/kg, and 295 mg/kg in the mouse and rat, respectively. 50
DOSAGE AND ADMINISTRATION
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Although intended primarily for administration to children, the syrup is also used for administration to adults who cannot swallow tablets.
The total daily dosage for children may be calculated on the basis of body weight or body area using approximately 0.25 mg/kg/day (0.11 mg/lb/day) or 8 mg per square meter of body surface (8 mg/m ). Children:2
The usual dose is 2 mg (one teaspoonful) two or three times a day, adjusted as necessary to the size and response of the patient. The dose is not to exceed 12 mg a day. Age 2 to 6 years:
The usual dose is 4 mg (two teaspoonsful) two or three times a day, adjusted as necessary to the size and response of the patient. The dose is not to exceed 16 mg a day. Age 7 to 14 years:
The total daily dose for adults should not exceed 0.5 mg/kg/day (0.23 mg/lb/day). The therapeutic range is 4 to 20 mg a day, with the majority of patients requiring 12 to 16 mg a day. An occasional patient may require as much as 32 mg a day for adequate relief. It is suggested that dosage be initiated with 4 mg (two teaspoonsful) three times a day and adjusted according to the size and response of the patient. Adults:
