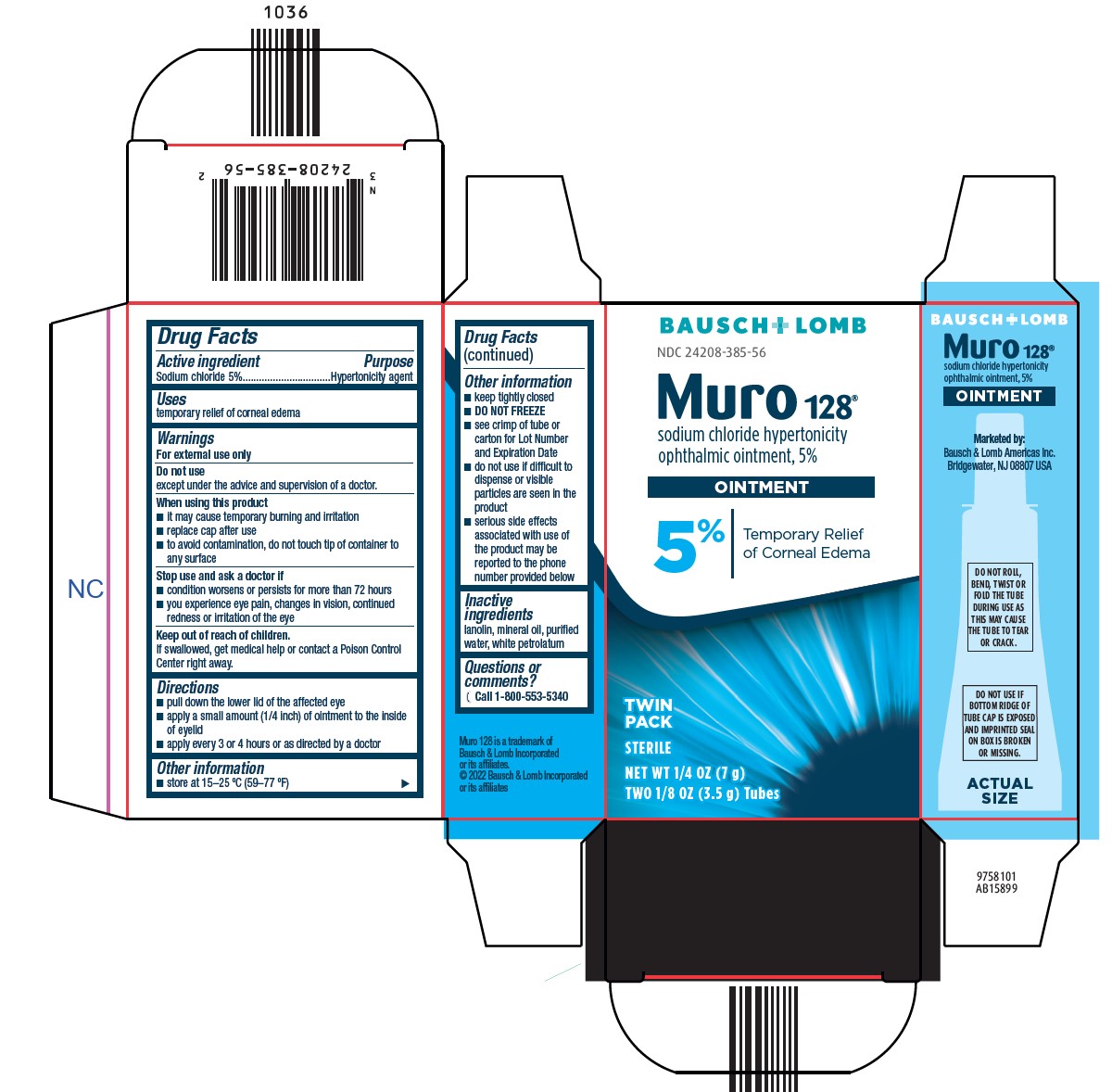
Warnings
For external use only
Do not use
except under the advice and supervision of a doctor.
When using this product
- •
- it may cause temporary burning and irritation
- •
- replace cap after use
- •
- to avoid contamination, do not touch tip of container to any surface
Stop use and ask a doctor if
- •
- condition worsens or persists for more than 72 hours
- •
- you experience eye pain, changes in vision, continued redness or irritation of the eye
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- pull down the lower lid of the affected eye
- •
- apply a small amount (1/4 inch) of ointment to the inside of eyelid
- •
- apply every 3 or 4 hours or as directed by a doctor
Other information
- •
- store at 15-25 °C (59-77 °F)
- •
- keep tightly closed
- •
- DO NOT FREEZE
- •
- see crimp of tube or carton for Lot Number and Expiration Date
- •
- do not use if difficult to dispense or visible particles are seen in the product
- •
- serious side effects associated with use of the product may be reported to the phone number provided below