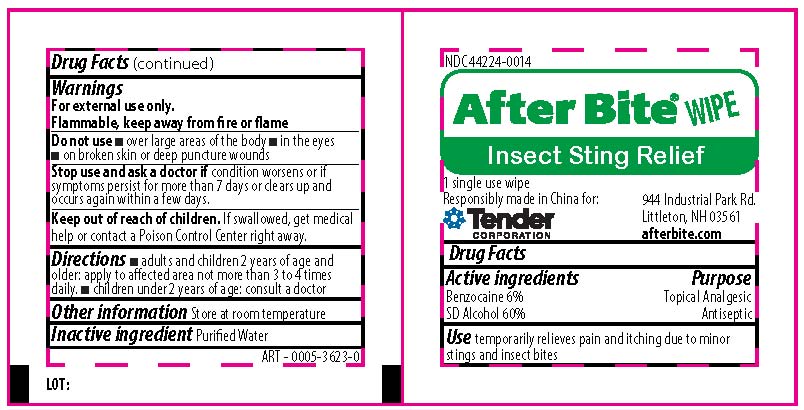
Stop use and ask a doctor if
- irritation and redness develop
- condition persists for more than 72 hours
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
ACTIVE INGREDIENTS
Bacitracin Zinc 400 units
Neomycin Sulfate 5mg ( equivalent to 3.5 mg Neomycin base)
Polymyxin B Sulfate 5000 units
STOP USE AND ASK A DOCTOR IF
Symptoms persist for more than 7 days or clear up and occur again within a few days.
KEEP OUT OF REACH OF CHILDREN
if swallowed, get medical help or contact a Poison Control Center right away
Directions
- adults and children 2 year of age and older: clean the affected area; apply a small amount to the area 1-3 times daily; may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
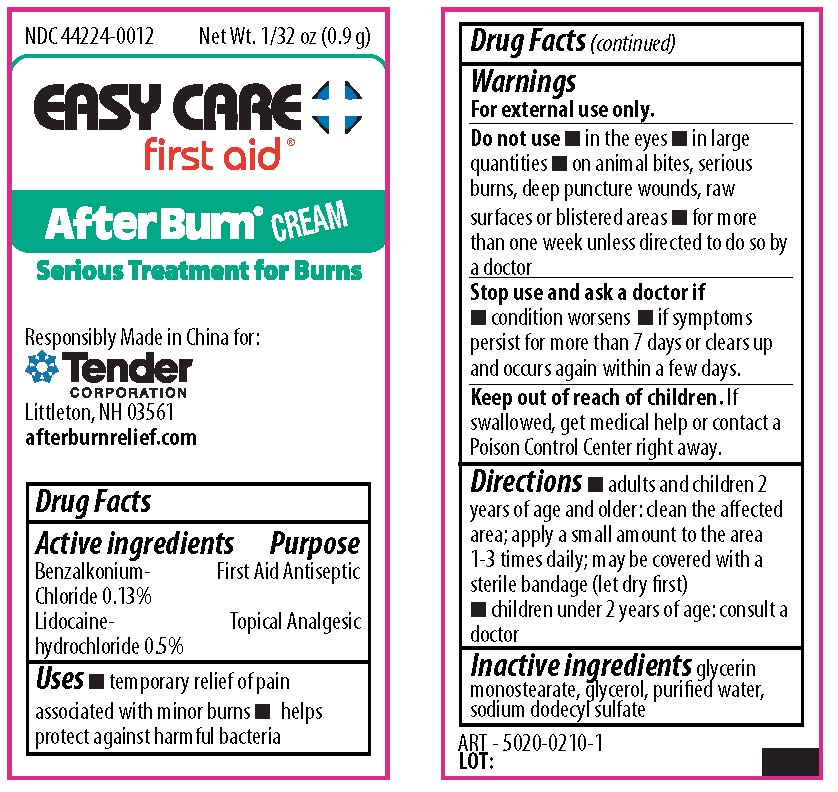
STOP USE AND ASK A DOCTOR IF
condition worsens or if symptoms persist for more than 7 days or clears up and occurs again within a few days.
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- adults and children 2 year of age and older: apply to affected area not more than 3 to 4 times daily.
- children under 2 years of age: consult a doctor
DO NOT USE
- in the eyes
- in large quanitites
- on animal bites, serious burns, deep puncture wounds, raw surfaces, or blistered areas
- for more than one week unless directed to do so by a doctor
STOP USE AND ASK A DOCTOR IF
- condition worsens
- if symptoms persist for more than 7 days or clears up and occurs again within a few days
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- adults and children 2 years of age and older: clean the affected area; apply a small amount to the area 1-3 times daily; may be covered with a sterile bandage (let dry first).
- children under 2 years of age: consult a doctor
Easy Care First Aid® Skin Cleansing Alcohol Wipe
Responsibly made in China for:
Tender Corporation
944 Industrial Park Rd.
Littleton, NH 03561
easycarefirstaid.com
1 single use wipe

PRINCIPAL DISPLAY PANEL
Easy Care First Aid
Triple Antibiotic Ointment
Responsibly Made in China for:
Tender Corporation
Littleton, NH 03561
easycarefirstaid.com