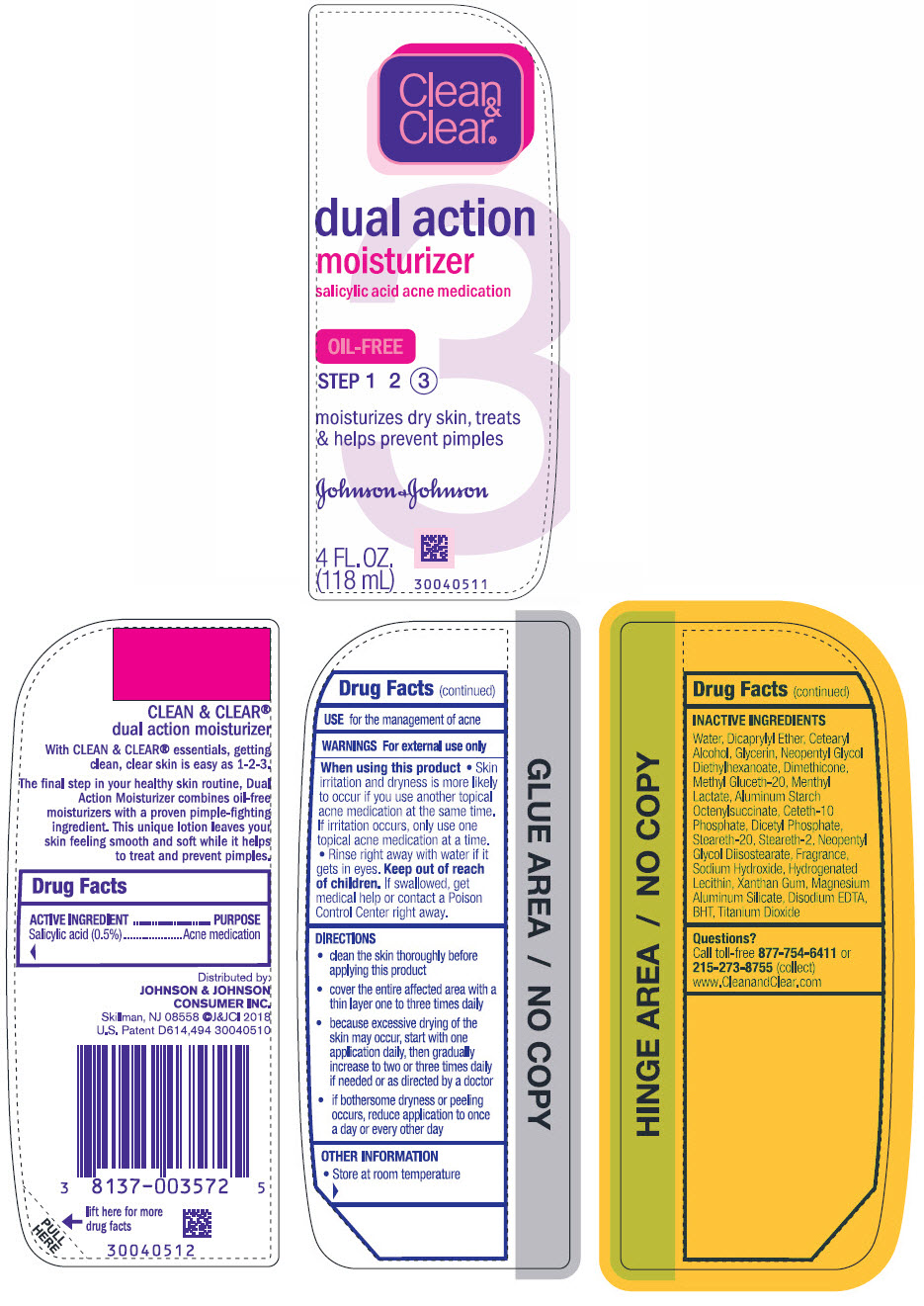
CLEAN AND CLEAR DUAL ACTION MOISTURIZER- salicylic acid lotion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic Acid (0.5%)
Use
for the management of acne
Warnings
For external use only.
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Rinse right away with water if it gets in eyes.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Other information
- Store at room temperature
Inactive ingredients
Water, Dicaprylyl Ether, Cetearyl Alcohol, Glycerin, Neopentyl Glycol, Diethylhexanoate, Dimethicone, Methyl Gluceth-20, Menthyl Lactate, Aluminum Starch Octenylsuccinate, Ceteth-10 Phosphate, Dicetyl Phosphate, Steareth-20, Steareth-2, Neopentyl Glycol Diisostearate, Fragrance, Sodium Hydroxide, Hydrogenated Lecithin, Xanthan Gum, Magnesium Aluminum Silicate, Disodium EDTA, BHT, Titanium Dioxide
Questions?
Call toll-free
877-754-6411 or 215-273-8755 (collect) www.CleanandClear.com
Distributed by:
JOHNSON & JOHNSON
CONSUMER INC.
Skillman, NJ 08558
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
Clean
&
Clear
®
dual action
moisturizer
salicylic acid acne medication
OIL-FREE
STEP 1 2 3
moisturizes dry skin, treats
& helps prevent pimples
Johnson & Johnson
4 FL. OZ.
(118mL)
Johnson & Johnson Consumer Inc.