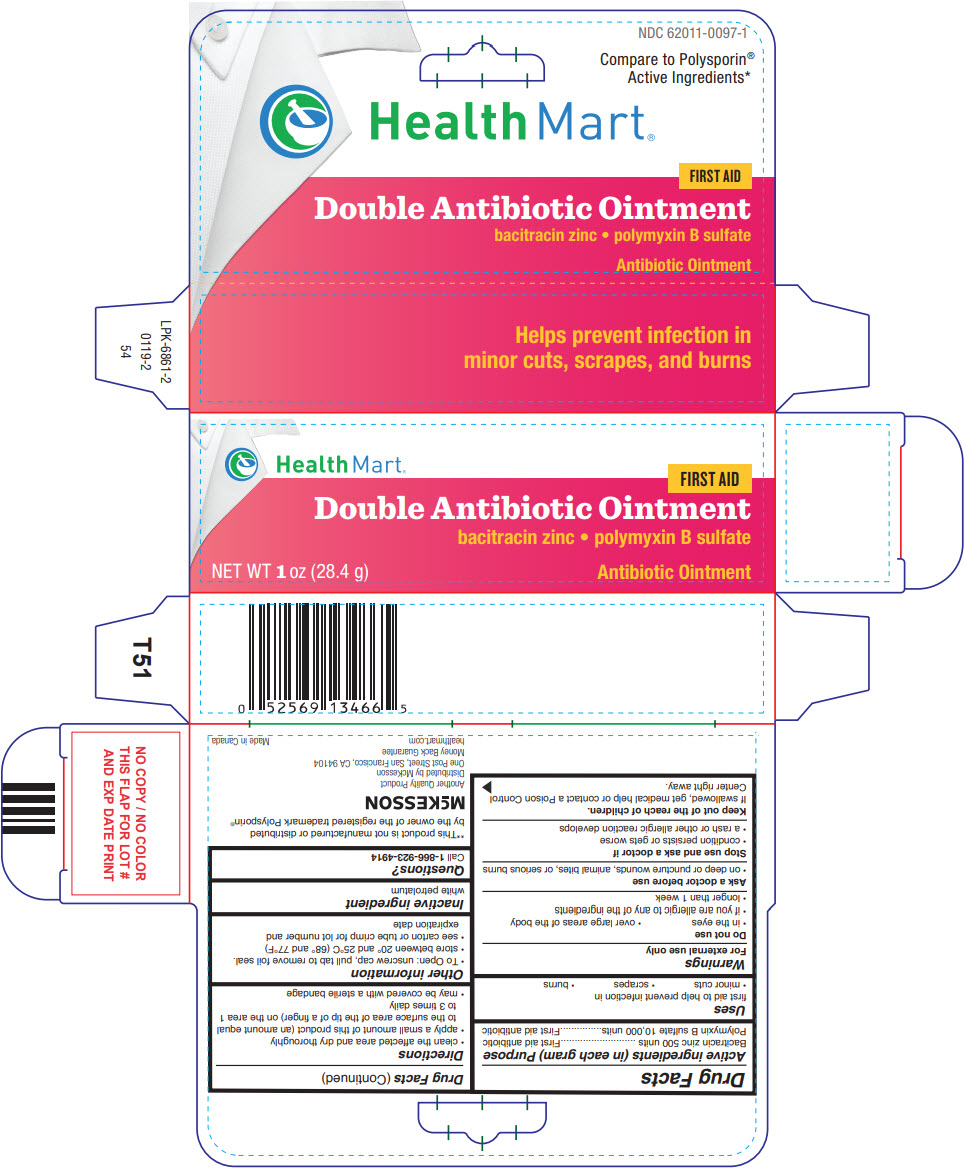
| Active ingredients (in each gram) | Purpose |
| Bacitracin zinc 500 units | First aid antibiotic |
| Polymyxin B sulfate 10,000 units | First aid antibiotic |
Uses
first aid to help prevent infection in
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
- if you are allergic to any of the ingredients
- longer than 1 week
Ask a doctor before use
- on deep or puncture wounds, animal bites, or serious burns
Stop use and ask a doctor if
- condition persists or gets worse
- a rash or other allergic reaction develops
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the affected area and dry thoroughly
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Other information
- To Open: unscrew cap, pull tab to remove foil seal.
- store between 20° and 25°C (68° and 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredient
white petrolatum
Questions?
Call
1-866-923-4914
Distributed by McKesson
One Post Street, San Francisco, CA 94104
PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
NDC 62011-0097-1
Compare to Polysporin
®
Active Ingredients*
HealthMart
®
FIRST AID
Double Antibiotic Ointment
bacitracin zinc • polymyxin B sulfate
NET WT
1oz (28.4 g)
Antibiotic Ointment