To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Hydrochloride for Injection, USP and other antibacterial drugs, Vancomycin Hydrochloride for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than a 500 mg dose of vancomycin.
Vancomycin Hydrochloride for Injection, USP is a white to almost white to brownish lyophilized powder for preparing intravenous (I.V.) infusions, in bags containing the equivalent of 100 grams vancomycin base. 500 mg of the base are equivalent to 0.34 mmol. When reconstituted with Sterile Water for Injection to a concentration of 100 mg/mL, a clear, brownish-yellow solution is formed with the pH of the solution between 2.5 and 4.5. This product is oxygen sensitive. Vancomycin Hydrochloride for Injection, USP should be administered intravenously in diluted solution (see DOSAGE AND ADMINISTRATION). AFTER RECONSTITUTION FURTHER DILUTION IS REQUIRED BEFORE USE.
Each SmartPak® Pharmacy Bulk Package contains Vancomycin Hydrochloride for Injection, USP equivalent to 100 grams of vancomycin activity and is intended for intravenous infusion only following further dilution. A Pharmacy Bulk Package is a sterile dosage form containing many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion.
VANCOMYCIN HYDROCHLORIDE FOR INJECTION PHARMACY BULK PACKAGE BAG SMARTPAK® SHOULD NOT BE USED IN PATIENTS WHO REQUIRE LESS THAN A 500 MG DOSE OF VANCOMYCIN.
BEFORE ADMINISTRATION, THIS PHARMACY BULK PACKAGE REQUIRES RECONSTITUTION USING STERILE WATER FOR INJECTION, USP TO A CONCENTRATION OF 100 MG PER ML AND FURTHER DILUTION IN 100 ML OF A COMPATIBLE SOLUTION AND INFUSED INTRAVENOUSLY.
THIS PRODUCT IS NOT INTENDED TO BE USED IN PEDIATRIC AND RENALLY IMPAIRED PATIENTS WHO REQUIRE LESS THAN A 500 MG DOSE.
Vancomycin is a tricyclic glycopeptide antibiotic derived from Amycolatopasis orientalis (formerly Nocardia orientalis). The chemical name for vancomycin hydrochloride is [3S- [3R*,6S*(S*),7S*,22S*,23R*,26R*,36S*,38aS*]]-3-(2-Amino-2-oxoethyl)-44-[[2-O-(3-amino- 2,3,6-trideoxy-3-C-methyl-α-L-lyxo-hexopyranosyl)-ß-D-glucopyranosyl]oxy]-10,19-dichloro- 2,3,4,5,6,7,23,24,25,26,36,37,38,38a-tetradecahydro-7,22,28,30,32-pentahydroxy-6-[[4-methyl- 2-(methylamino)-1-oxopentyl]amino]-2,5,24,38,39-pentaoxo-22H-8,11:18,21-dietheno-23,36- (iminomethano)-13,16:31,35-dimetheno-1H,16H-[1,6,9]oxadiazacyclohexadecino[4,5- m][10,2,16]-benzoxadiazacyclotetracosine-26-carboxylic acid, monohydrochloride. The molecular formula is C66H75Cl2N9O24• HCl and the molecular weight is 1,485.74.
Vancomycin Hydrochloride has the following structural formula:
CLINICAL PHARMACOLOGY
Vancomycin is poorly absorbed after oral administration.
In subjects with normal kidney function, multiple intravenous dosing of 1 g of vancomycin (15 mg/kg) infused over 60 minutes produces mean plasma concentrations of approximately 63 mcg/mL immediately after the completion of infusion, mean plasma concentrations of approximately 23 mcg/mL 2 hours after infusion, and mean plasma concentrations of approximately 8 mcg/mL 11 hours after the end of the infusion. Multiple dosing of 500 mg infused over 30 minutes produces mean plasma concentrations of about 49 mcg/mL at the completion of infusion, mean plasma concentrations of about 19 mcg/mL 2 hours after infusion, and mean plasma concentrations of about 10 mcg/mL 6 hours after infusion. The plasma concentrations during multiple dosing are similar to those after a single dose.
The mean elimination half-life of vancomycin from plasma is 4 to 6 hours in subjects with normal renal function. In the first 24 hours, about 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration. Mean plasma clearance is about 0.058 L/kg/h, and mean renal clearance is about 0.048 L/kg/h. Renal dysfunction slows excretion of vancomycin. In anephric patients, the average half-life of elimination is 7.5 days. The distribution coefficient is from 0.3 to 0.43 L/kg. There is no apparent metabolism of the drug. About 60% of an intraperitoneal dose of vancomycin administered during peritoneal dialysis is absorbed systemically in 6 hours. Serum concentrations of about 10 mcg/mL are achieved by intraperitoneal injection of 30 mg/kg of vancomycin. However, the safety and efficacy of the intraperitoneal use of vancomycin has not been established in adequate and well-controlled trials (see PRECAUTIONS).
Total systemic and renal clearance of vancomycin may be reduced in the elderly.
Vancomycin is approximately 55% serum protein bound as measured by ultrafiltration at vancomycin serum concentrations of 10 to 100 mcg/mL. After IV administration of vancomycin, inhibitory concentrations are present in pleural, pericardial, ascitic, and synovial fluids; in urine; in peritoneal dialysis fluid; and in atrial appendage tissue. Vancomycin does not readily diffuse across normal meninges into the spinal fluid; but, when the meninges are inflamed, penetration into the spinal fluid occurs.
MICROBIOLOGY
The bactericidal action of vancomycin results primarily from inhibition of cell-wall biosynthesis. In addition, vancomycin alters bacterial-cell-membrane permeability and RNA synthesis. There is no cross-resistance between vancomycin and other antibiotics. Vancomycin is not active in vitro against gram-negative bacilli, mycobacteria, or fungi.
Synergy
The combination of vancomycin and an aminoglycoside acts synergistically in vitro against many strains of Staphylococcus aureus, Streptococcus bovis, enterococci, and the viridans group streptococci.
Vancomycin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-positive microorganisms
Diphtheroids
Enterococci (e.g., Enterococcus faecalis)
Staphylococci, including Staphylococcus aureus and Staphylococcus epidermidis (including heterogeneous methicillin-resistant strains)
Streptococcus bovis
Viridans group streptococci
The following in vitro data are available, but their clinical significance is unknown.
Vancomycin exhibits in vitro MIC’s of 1 mcg/mL or less against most (≥90%) strains of streptococci listed below and MIC’s of 4 mcg/mL or less against most (≥90%) strains of other listed microorganisms; however the safety and effectiveness of vancomycin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic gram-positive microorganisms
Listeria monocytogenes
Streptococcus pyogenes
Streptococcus pneumoniae (including penicillin-resistant strains)
Streptococcus agalactiae
Anaerobic gram-positive microorganisms
Actinomyces species
Lactobacillus species
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
INDICATIONS AND USAGE
Vancomycin Hydrochloride for Injection, USP is indicated for the treatment of serious or severe infections caused by susceptible strains of methicillin-resistant (beta-lactam-resistant) staphylococci. It is indicated for penicillin-allergic patients, for patients who cannot receive or who have failed to respond to other drugs, including the penicillins or cephalosporins, and for infections caused by vancomycin-susceptible organisms that are resistant to other antimicrobial drugs. Vancomycin is indicated for initial therapy when methicillin-resistant staphylococci are suspected, but after susceptibility data are available, therapy should be adjusted accordingly.
Vancomycin is effective in the treatment of staphylococcal endocarditis. Its effectiveness has been documented in other infections due to staphylococci, including septicemia, bone infections, lower respiratory tract infections, and skin and skin-structure infections. When staphylococcal infections are localized and purulent, antibiotics are used as adjuncts to appropriate surgical measures.
Vancomycin has been reported to be effective alone or in combination with an aminoglycoside for endocarditis caused by Streptococcus viridans or S. bovis. For endocarditis caused by enterococci (e.g., E. faecalis), vancomycin has been reported to be effective only in combination with an aminoglycoside.
Vancomycin has been reported to be effective for the treatment of diphtheroid endocarditis. Vancomycin has been used successfully in combination with either rifampin, an aminoglycoside, or both in early-onset prosthetic valve endocarditis caused by S. epidermidis or diphtheroids.
Specimens for bacteriologic cultures should be obtained in order to isolate and identify causative organisms and to determine their susceptibilities to vancomycin.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Hydrochloride for Injection, USP and other antibacterial drugs, vancomycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
The parenteral form of vancomycin hydrochloride may be administered orally for treatment of antibiotic-associated pseudomembranous colitis produced by C. difficile and for staphylococcal enterocolitis. Parenteral administration of vancomycin hydrochloride alone is of unproven benefit for these indications. Vancomycin is not effective by the oral route for other types of infection.
CONTRAINDICATIONS
Vancomycin hydrochloride for injection is contraindicated in patients with known hypersensitivity to this antibiotic.
WARNINGS
Infusion Reactions
Rapid bolus administration (e.g., over several minutes) may be associated with exaggerated hypotension, including shock and rarely cardiac arrest. Vancomycin hydrochloride for injection should be administered in a diluted solution over a period of not less than 60 minutes to avoid rapid-infusion-related reactions. Stopping the infusion usually results in prompt cessation of these reactions.
Nephrotoxicity
This formulation of Vancomycin Hydrochloride for Injection, USP, Pharmacy Bulk Package bags SmartPak® should not be used for renally impaired patients who require less than a 500 mg dose of vancomycin.
Systemic vancomycin exposure may result in acute kidney injury (AKI). The risk of AKI increases as systemic exposure/serum levels increase. Monitor renal function in all patients receiving vancomycin, especially patients with underlying renal impairment, patients with co-morbidities that predispose to renal impairment, and patients receiving concomitant therapy with a drug known to be nephrotoxic.
Ototoxicity
Ototoxicity has occurred in patients receiving vancomycin hydrochloride for injection. It may be transient or permanent. It has been reported mostly in patients, who have been given excessive doses, who have an underlying hearing loss, or who are receiving concomitant therapy with another ototoxic agent, such as an aminoglycoside. Vancomycin should be used with caution in patients with renal insufficiency because the risk of toxicity is appreciably increased by high, prolonged blood concentrations.
Dosage of vancomycin hydrochloride for injection must be adjusted for patients with renal dysfunction (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Clostridium Difficile Associated Diarrhea (CDAD)
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including vancomycin hydrochloride for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Hemorrhagic Occlusive Retinal Vasculitis (HORV)
Hemorrhagic occlusive retinal vasculitis, including permanent loss of vision, occurred in patients receiving intracameral or intravitreal administration of vancomycin during or after cataract surgery. The safety and efficacy of vancomycin administered by the intracameral or the intravitreal route have not been established by adequate and well-controlled trials. Vancomycin is not indicated for the prophylaxis of endophthalmitis.
PRECAUTIONS
Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin.
Prolonged use of vancomycin hydrochloride for injection may result in the overgrowth of nonsusceptible microorganisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken. In rare instances, there have been reports of pseudomembranous colitis due to C. difficile developing in patients who received intravenous vancomycin hydrochloride for injection.
Serial tests of auditory function may be helpful in order to minimize the risk of ototoxicity.
Reversible neutropenia has been reported in patients receiving vancomycin hydrochloride for injection (see ADVERSE REACTIONS). Patients who will undergo prolonged therapy with vancomycin hydrochloride for injection or those who are receiving concomitant drugs which may cause neutropenia should have periodic monitoring of the leukocyte count.
Vancomycin hydrochloride for injection is irritating to tissue and must be given by a secure IV route of administration. Pain, tenderness, and necrosis occur with intramuscular (IM) injection of vancomycin hydrochloride for injection or with inadvertent extravasation. Thrombophlebitis may occur, the frequency and severity of which can be minimized by administering the drug slowly as a dilute solution (2.5 to 5 grams/L) and by rotation of the venous access sites.
There have been reports that the frequency of infusion-related events (including hypotension, flushing, erythema, urticaria, and pruritus) increases with the concomitant administration of anesthetic agents. Infusion-related events may be minimized by the administration of vancomycin as a 60-minute infusion prior to anesthetic induction. The safety and efficacy of vancomycin administered by the intrathecal (intralumbar or intraventricular) route or by the intraperitoneal route have not been established by adequate and well-controlled trials.
Reports have revealed that administration of sterile vancomycin by the intraperitoneal route during continuous ambulatory peritoneal dialysis (CAPD) has resulted in a syndrome of chemical peritonitis. To date, this syndrome has ranged from cloudy dialysate alone to a cloudy dialysate accompanied by variable degrees of abdominal pain and fever. This syndrome appears to be short-lived after discontinuation of intraperitoneal vancomycin.
Prescribing vancomycin hydrochloride for injection, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Drug Interactions
Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing (see PRECAUTIONS, Pediatric Use) and anaphylactoid reactions (see ADVERSE REACTIONS).
Monitor renal function in patients receiving vancomycin and concurrent and/or sequential systemic or topical use of other potentially, neurotoxic and/or nephrotoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymyxin B, colistin, viomycin, or cisplatin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Although no long-term studies in animals have been performed to evaluate carcinogenic potential, no mutagenic potential of vancomycin hydrochloride for injection was found in standard laboratory tests. No definitive fertility studies have been performed.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with vancomycin. It is not known whether vancomycin can affect reproduction capacity. In a controlled clinical study, the potential ototoxic and nephrotoxic effects of vancomycin on infants were evaluated when the drug was administered to pregnant women for serious staphylococcal infections complicating intravenous drug abuse. Vancomycin was found in cord blood. No sensorineural hearing loss or nephrotoxicity attributable to vancomycin was noted. One infant whose mother received vancomycin in the third trimester experienced conductive hearing loss that was not attributed to the administration of vancomycin. Because the number of patients treated in this study was limited and vancomycin was administered only in the second and third trimesters, it is not known whether vancomycin causes fetal harm. Vancomycin should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Vancomycin hydrochloride for injection is excreted in human milk. Caution should be exercised when vancomycin hydrochloride for injection is administered to a nursing woman. Because of the potential for adverse events, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in pediatric patients who require less than a 500 mg dose of vancomycin. In pediatric patients, it may be appropriate to confirm desired vancomycin serum concentrations. Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing in pediatric patients (see PRECAUTIONS).
Geriatric Use
The natural decrement of glomerular filtration with increasing age may lead to elevated vancomycin serum concentrations if dosage is not adjusted. Vancomycin dosage schedules should be adjusted in elderly patients (see DOSAGE AND ADMINISTRATION).
Information for Patients
Patients should be counseled that antibacterial drugs including vancomycin hydrochloride for injection, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When vancomycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by vancomycin hydrochloride for injection or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
ADVERSE REACTIONS
Infusion-Related Events
During or soon after rapid infusion of vancomycin hydrochloride for injection, patients may develop anaphylactoid reactions, including hypotension (see ANIMAL PHARMACOLOGY), wheezing, dyspnea, urticaria, or pruritus. Rapid infusion may also cause flushing of the upper body (“red neck”) or pain and muscle spasm of the chest and back. These reactions usually resolve within 20 minutes but may persist for several hours. Such events are infrequent if vancomycin hydrochloride for injection is given by a slow infusion over 60 minutes. In studies of normal volunteers, infusion-related events did not occur when vancomycin hydrochloride for injection was administered at a rate of 10 mg/minute or less.
Nephrotoxicity
Systemic vancomycin exposure may result in acute kidney injury (AKI). The risk of AKI increases as systemic exposure/serum levels increase. Additional risk factors for AKI in patients receiving vancomycin include receipt of concomitant drugs known to be nephrotoxic, in patients with pre-existing renal impairment, or with co-morbidities that predispose to renal impairment. Interstitial nephritis has also been reported in patients receiving vancomycin.
Gastrointestinal
Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment (see WARNINGS).
Ototoxicity
A few dozen cases of hearing loss associated with vancomycin have been reported. Most of these patients had kidney dysfunction or a preexisting hearing loss or were receiving concomitant treatment with an ototoxic drug. Vertigo, dizziness, and tinnitus have been reported rarely.
Hematopoietic
Reversible neutropenia, usually starting 1 week or more after onset of therapy with vancomycin or after a total dosage of more than 25 g, has been reported for several dozen patients. Neutropenia appears to be promptly reversible when vancomycin is discontinued. Thrombocytopenia has rarely been reported. Although a causal relationship has not been established, reversible agranulocytosis (granulocytes <500/mm3) has been reported rarely.
Miscellaneous
Infrequently, patients have been reported to have had anaphylaxis, drug fever, nausea, chills, eosinophilia, rashes including exfoliative dermatitis, linear IgA bullous dermatosis, Stevens-Johnson syndrome, toxic epidermal necrolysis and vasculitis in association with administration of vancomycin. Chemical peritonitis has been reported following intraperitoneal administration of vancomycin (see PRECAUTIONS).
POST MARKETING REPORTS
The following adverse reactions have been identified during post-approval use of vancomycin. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders
Drug Rash with Eosinophilia and Systemic Symptoms (DRESS)
To report SUSPECTED ADVERSE EVENTS, contact Samson Medical Technologies, L.L.C. at 1-877-418-3600 or FDA at 1-800-FDA-1088 or http://www.fda.gov/ for voluntary reporting of adverse reactions.
OVERDOSAGE
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to result in increased vancomycin clearance. The median lethal intravenous dose is 319 mg/kg in rats and 400 mg/kg in mice.
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
DOSAGE AND ADMINISTRATION
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than a 500 mg dose of vancomycin.
The intent of the pharmacy bulk package for this product is for preparation of solutions for intravenous infusion only.
THIS PHARMACY BULK PACKAGE REQUIRES RECONSTITUTION WITH STERILE WATER FOR INJECTION, USP TO A CONCENTRATION OF 100 mg per mL AND FURTHER DILUTION IN 100 mL OF A COMPATIBLE SOLUTION.
Infusion-related events are related to both the concentration and the rate of administration of vancomycin. Concentrations of no more than 5 mg/mL and rates of no more than 10 mg/minute are recommended in adults (see also age-specific recommendations). In selected patients in need of fluid restriction, a concentration of up to 10 mg/mL may be used; use of such higher concentrations may increase the risk of infusion-related events. An infusion rate of 10 mg/min or less is associated with fewer infusion-related events (see ADVERSE REACTIONS). Infusion-related events may occur, however, at any rate or concentration.
Patients with Normal Renal Function
Adults
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package SmartPak® should not be used in patients who require less than a 500 mg dose of vancomycin. The usual daily intravenous dose is 2 g divided either as 500 mg every 6 hours or 1 g every 12 hours. Each dose should be administered at no more than 10 mg/minute or over a period of at least 60 minutes, whichever is longer. Other patient factors, such as age or obesity, may call for modification of the usual intravenous daily dose.
Pediatric patients
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package SmartPak® should not be used in pediatric patients who require less than a 500 mg dose of vancomycin. The usual intravenous dosage of vancomycin is 10 mg/kg per dose given every 6 hours. Each dose should be administered over a period of at least 60 minutes.
Close monitoring of serum concentrations of vancomycin may be warranted in these patients.
Neonates
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package SmartPak® should not be used in pediatric patients who require less than a 500 mg dose of vancomycin. In pediatric patients up to the age of 1 month, the total daily intravenous dosage may be lower. In neonates, an initial dose of 15 mg/kg is suggested, followed by 10 mg/kg every 12 hours for neonates in the 1st week of life and every 8 hours thereafter up to the age of 1 month. Each dose should be administered over 60 minutes. In premature infants, vancomycin clearance decreases as postconceptional age decreases. Therefore, longer dosing intervals may be necessary in premature infants. Close monitoring of serum concentrations of vancomycin is recommended in these patients.
Patients with Impaired Renal Function and Elderly Patients
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package SmartPak® should not be used in patients with renal impairment who require less than a 500 mg dose of vancomycin.
Dosage adjustment must be made in patients with impaired renal function. In premature infants and the elderly, greater dosage reductions than expected may be necessary because of decreased renal function. Measurement of vancomycin serum concentrations can be helpful in optimizing therapy, especially in seriously ill patients with changing renal function. Vancomycin serum concentrations can be determined by use of microbiologic assay, radioimmunoassay, fluorescence polarization immunoassay, fluorescence immunoassay or high-pressure liquid chromatography.
If creatinine clearance can be measured or estimated accurately, the dosage for most patients with renal impairment can be calculated using the following table. The dosage of vancomycin hydrochloride for injection per day in mg is about 15 times the glomerular filtration rate in mL/minute (see following table).
The initial dose should be no less than 15 mg/kg, even in patients with mild to moderate renal insufficiency.
The table is not valid for functionally anephric patients. For such patients, an initial dose of 15 mg/kg of body weight should be given to achieve prompt therapeutic serum concentrations. The dose required to maintain stable concentrations is 1.9 mg/kg/24 hr. In patients with marked renal impairment, it may be more convenient to give maintenance doses of 250 to 1,000 mg once every several days rather than administering the drug on a daily basis. In anuria, a dose of 1,000 mg every 7 to 10 days has been recommended.
When only serum creatinine concentration is known, the following formula (based on sex, weight and age of the patient) may be used to calculate creatinine clearance. Calculated creatinine clearances (mL/min) are only estimates. The creatinine clearance should be measured promptly.
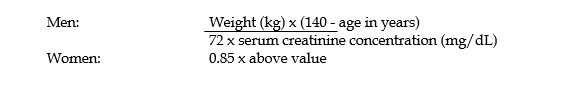
The serum creatinine must represent a steady state of renal function. Otherwise, the estimated value for creatinine clearance is not valid. Such a calculated clearance is an overestimate of actual clearance in patients with conditions: (1) characterized by decreasing renal function, such as shock, severe heart failure, or oliguria; (2) in which a normal relationship between muscle mass and total body weight is not present, such as in obese patients or those with liver disease, edema, or ascites; and (3) accompanied by debilitation, malnutrition, or inactivity. The safety and efficacy of vancomycin administration by the intrathecal (intralumbar or intraventricular) routes have not been established. Intermittent infusion is the recommended method of administration
Compatibility with Other Drugs and Intravenous Fluids
The following diluents are physically and chemically compatible (with 4 grams/L vancomycin hydrochloride):
5% Dextrose Injection, USP
0.9% Sodium Chloride Injection, USP
Good professional practice suggests that compounded admixtures should be administered as soon after preparation as is feasible.
Vancomycin solution has a low pH and may cause physical instability of other compounds.
Mixtures of solutions of vancomycin and beta-lactam antibiotics have been shown to be physically incompatible. The likelihood of precipitation increases with higher concentrations of vancomycin. It is recommended to adequately flush the intravenous lines between the administration of these antibiotics. It is also recommended to dilute solutions of vancomycin to 5 mg/mL or less.
Although intravitreal injection is not an approved route of administration for vancomycin, precipitation has been reported after intravitreal injection of vancomycin and ceftazidime for endophthalmitis using different syringes and needles. The precipitates dissolved gradually, with complete clearing of the vitreous cavity over two months and with improvement of visual acuity.
Preparation of Solution
Vancomycin Hydrochloride for Injection USP, Pharmacy Bulk Package SmartPak® should not be used in patients who require less than a 500 mg dose of vancomycin.
Directions for Proper Use of a Pharmacy Bulk Package
Following is a table provided for convenience in reconstituting Vancomycin Hydrochloride for Injection SmartPak® Pharmacy Bulk Package for intravenous administration:
|
SmartPak® Bag Size |
Amount of Sterile Water for Injection |
Approximate Concentration |
|
100 grams |
950 mL |
100 mg/mL (500 mg/5 mL) |
- NOT FOR DIRECT INFUSION. The Pharmacy Bulk Package is for use in the hospital pharmacy admixture service only in a suitable work area, such as a laminar flow hood. Using aseptic technique, the container closure may be penetrated only one time after reconstitution using a suitable sterile dispensing set or transfer device that allows measured dispensing of the contents. Use of a syringe and needle is not recommended as it may cause leakage. The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of 4 HOURS from initial reconstitution port closure entry is permitted to complete fluid transfer operations. This time limit should begin with the introduction of the solvent or diluent into the Pharmacy Bulk Package. Discard any unused portion after 4 HOURS. This pharmacy bulk package is not intended to be dispensed as a unit.
- PRIOR TO RECONSTITUTION: Visually examine outer (natural foil) bag for damage. IF THE SEAL IS BROKEN OR DAMAGE IS OBSERVED, DO NOT OPEN THE OUTER BAG. STERILITY OF THE INNER BAG SURFACE MAY BE COMPROMISED. DISCARD BOTH BAGS IMMEDIATELY. DO NOT USE THE INNER BAG IF PARTICULATE OR FOREIGN MATTER IS PRESENT, IF THE DRY POWDER IS BROWN, IF THE SEALS ARE NOT INTACT, OR IF THERE IS ANY OTHER DAMAGE TO THE BAG. IN SUCH CASES, DISCARD THE BAG IMMEDIATELY.
- After initial reconstitution port entry, use entire contents of the Pharmacy Bulk Package promptly. Any unused portion must be discarded after 4 HOURS.
- Gather the following items prior to the reconstitution of the product: Appropriate number of bags of Sterile Water for Injection and, depending upon the method of filling, appropriate sterile tubing and adapters.
INSTRUCTION FOR RECONSTITUTION OF THE PHARMACY BULK PACKAGE BAG SmartPak®
The entire contents of the bag and the preparation process (reconstitution and dilution) should be completed within 4 hours of initial entry.
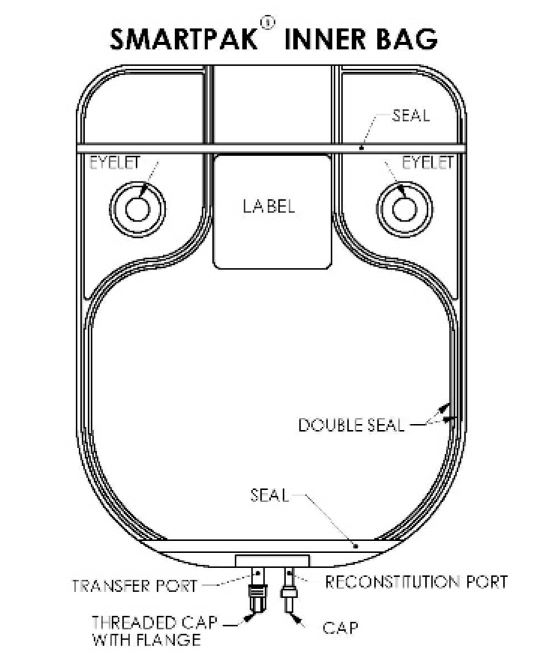
- Document the date and time reconstitution starts in the designated place on the container label. The entire contents of the bag must be used within 4 hours from the time of initial entry.
- Remove the translucent unthreaded cap from the reconstitution (smaller) port and discard it.
- Reconstitute the powder through the reconstitution (smaller) port, using Sterile Water for Injection according to the table shown under Preparation of Solution.
- After reconstitution is complete, remove the transfer needle from the reconstitution port.
- Place the bag on a flat surface of a laminar flow hood and mix for at least 20 minutes by rocking gently from side to side. CAUTION: To avoid possible leakage caused by the heavy weight of the added water, do not shake vigorously or pull strongly on the bag.
- When foam dissipates, visually inspect the bag to verify the solution is clear, colorless to pale yellow and free of particulate matter. DO NOT USE THE INNER BAG IF PARTICULATE OR FOREIGN MATTER IS PRESENT.
- Unscrew the clear threaded cap from the transfer (larger) port and discard it. Attach sterile tubing and filling adapter unit to the transfer port.
- Reconstituted solution can now be transferred using the transfer port and the filling adapter.
It should be noted that the spike placed into the transfer port of the Pharmacy Bulk Package SmartPak® is NEVER removed during this procedure and that the reconstitution port is self-sealing.
Dilution
- Hang the bag from two eyelets.
- Following reconstitution, transfer 5 mL of the reconstituted solution into transfusion bags, each containing 100 mL, of one of the compatible solutions below.
Compatible solutions for dilution are the following:
0.9% Sodium Chloride Injection, USP
5% Dextrose Injection, USP
- Dilution should be completed within the 4 hour preparation process.
- Reconstituted solutions containing 500 mg/5 mL (100 mg/mL) must be diluted in at least 100 mL of a suitable infusion solution. The desired dose, diluted in this manner, should be administered by intermittent IV infusion over a period of at least 60 minutes.
- Solutions that are diluted with 5% Dextrose Injection or 0.9% Sodium Chloride Injection may be stored in a refrigerator for 14 days without significant loss of potency.
Administration
Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration, whenever solution and container permit.
For Oral Administration
Oral vancomycin is used in treating antibiotic-associated pseudomembranous colitis caused by C. difficile and for staphylococcal enterocolitis. Vancomycin is not effective by the oral route for other types of infections. The usual adult total daily dosage is 500 mg to 2 g, given in 3 or 4 divided doses for 7 to 10 days. The total daily dose in children is 40 mg/kg of body weight in 3 or 4 divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. The appropriate dose may be diluted in 1 oz of water and given to the patients to drink. Common flavoring syrups may be added to the solution to improve the taste for oral administration. The diluted solution may be administered via a nasogastric tube.
HOW SUPPLIED
Vancomycin Hydrochloride for Injection, USP is a sterile, lyophilized powder available in the following SmartPak® Pharmacy Bulk Package:
100 grams* (1 Pharmacy Bulk Package) Product No. 7100 NDC 66288-7100-1 sold in individual bags.
*Each 100 gram pharmacy bulk package contains sterile vancomycin hydrochloride equivalent to 100 grams of vancomycin
Prior to reconstitution, Vancomycin Hydrochloride for Injection, USP should be stored at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
SmartPak® system components are not made with natural rubber latex.
ANIMAL PHARMACOLOGY
In animal studies, hypotension and bradycardia occurred in dogs receiving an intravenous infusion of vancomycin hydrochloride, 25 mg/kg, at a concentration of 25 mg/mL and an infusion rate of 13.3 mL/minute.
REFERENCES
- Moellering RC, Krogstad DJ, Greenblatt DJ: Vancomycin therapy in patients with impaired renal function: A nomogram for dosage. Ann Inter Med 1981; 94:343.
Revised 12/2018
C7100b
Manufactured for:
Samson Medical Technologies, L.L.C.
Cherry Hill, NJ 08003
Manufactured by:
Xellia Pharmaceuticals ApS
Copenhagen, Denmark
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Inner Bag Label
NDC 66288-7100-1 Smartpak®
Vancomycin Hydrochloride for Injection, USP
100 grams [ONE HUNDRED GRAMS]* per Pharmacy Bulk Package Bag
PHARMACY BULK PACKAGE -
NOT FOR DIRECT INFUSION
FOR INTRAVENOUS USE ONLY
NOT TO BE DISPENSED AS A UNIT
Rx only
