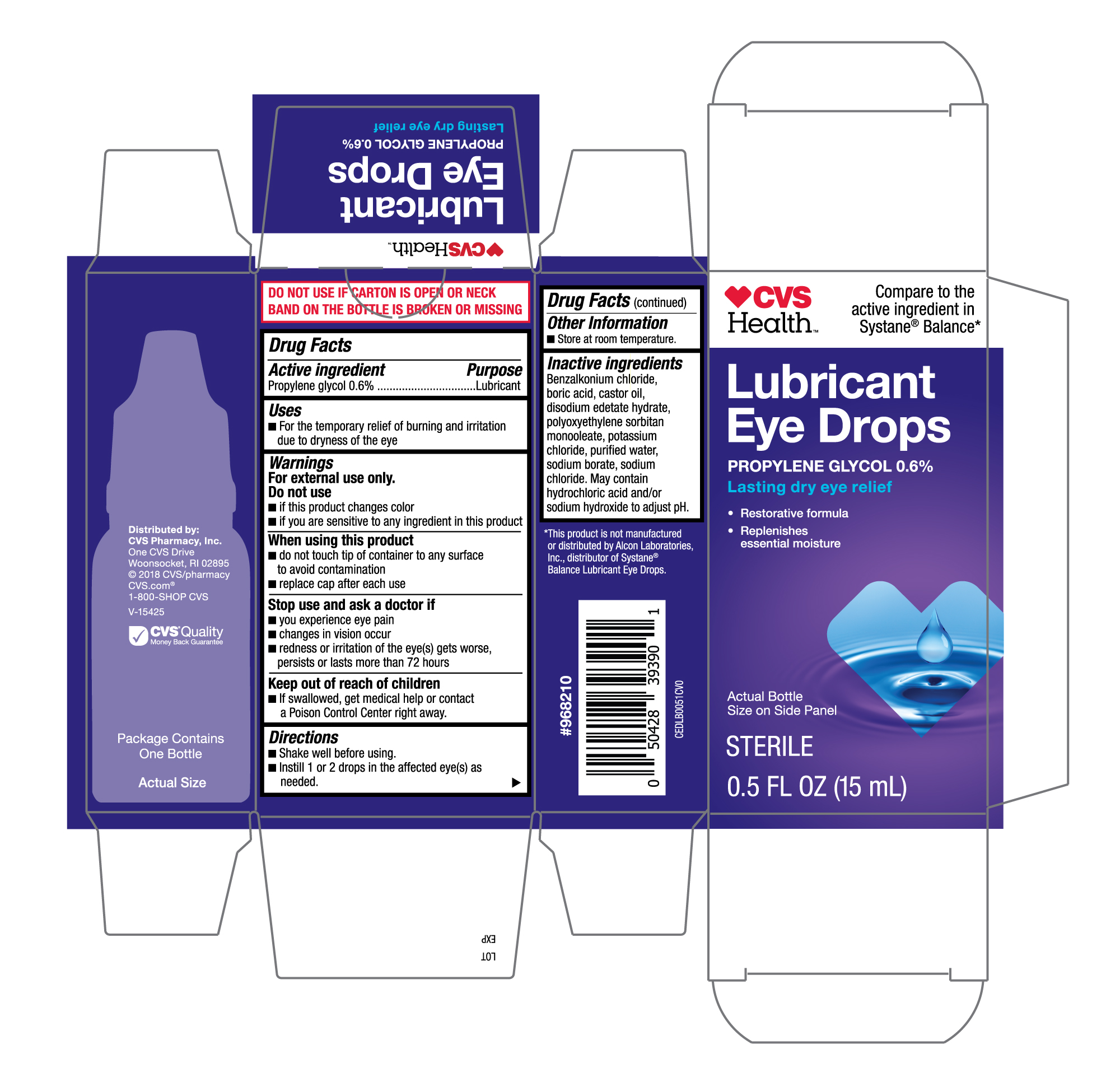
Active ingredient
Propylene glycol 0.6%
Uses
- For the temporary relief of burning and irritaion due to dryness of the eye
Warnings
For external use only.
Do not use
- if this product changes color
- if you are sensative to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you experience eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse, persists or lasts more than 72 hours
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake well befor using.
- instill 1 or 2 drops in the affected eye(s) as needed.
Other information
- Store at room temperature.
Inactive ingredients
Benzalkonium chloride, boric acid, castor oil, disodium edetate hydrate, polyoxyethylene sorbitan monooleate, potassium chloride, purified water, sodium borate, sodium chloride. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
CVS Lubricant Eye Drops 15mL
CVS Lubricant Eye Drops 15mL twin pack

CVS Pharmacy, Inc.