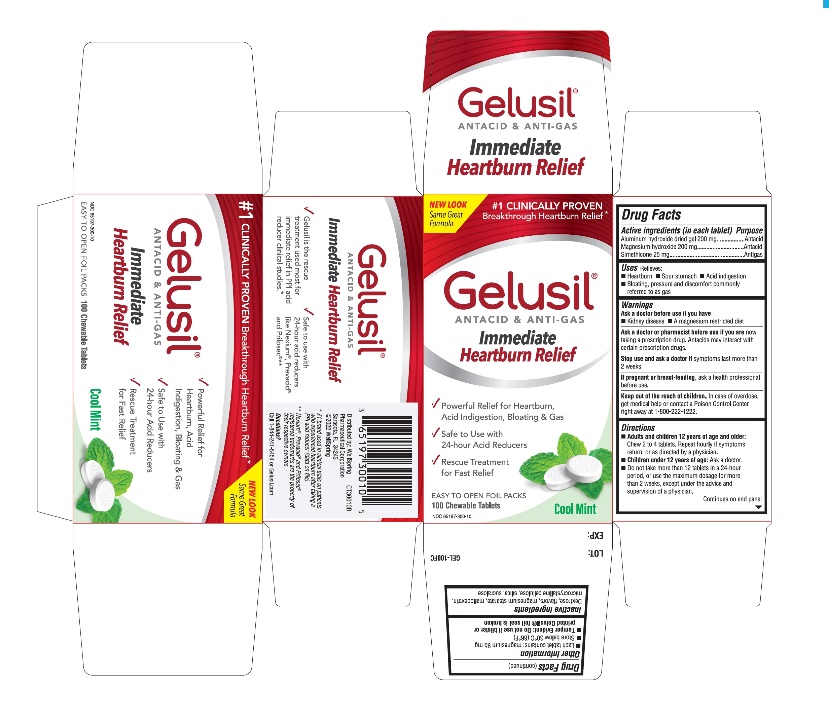
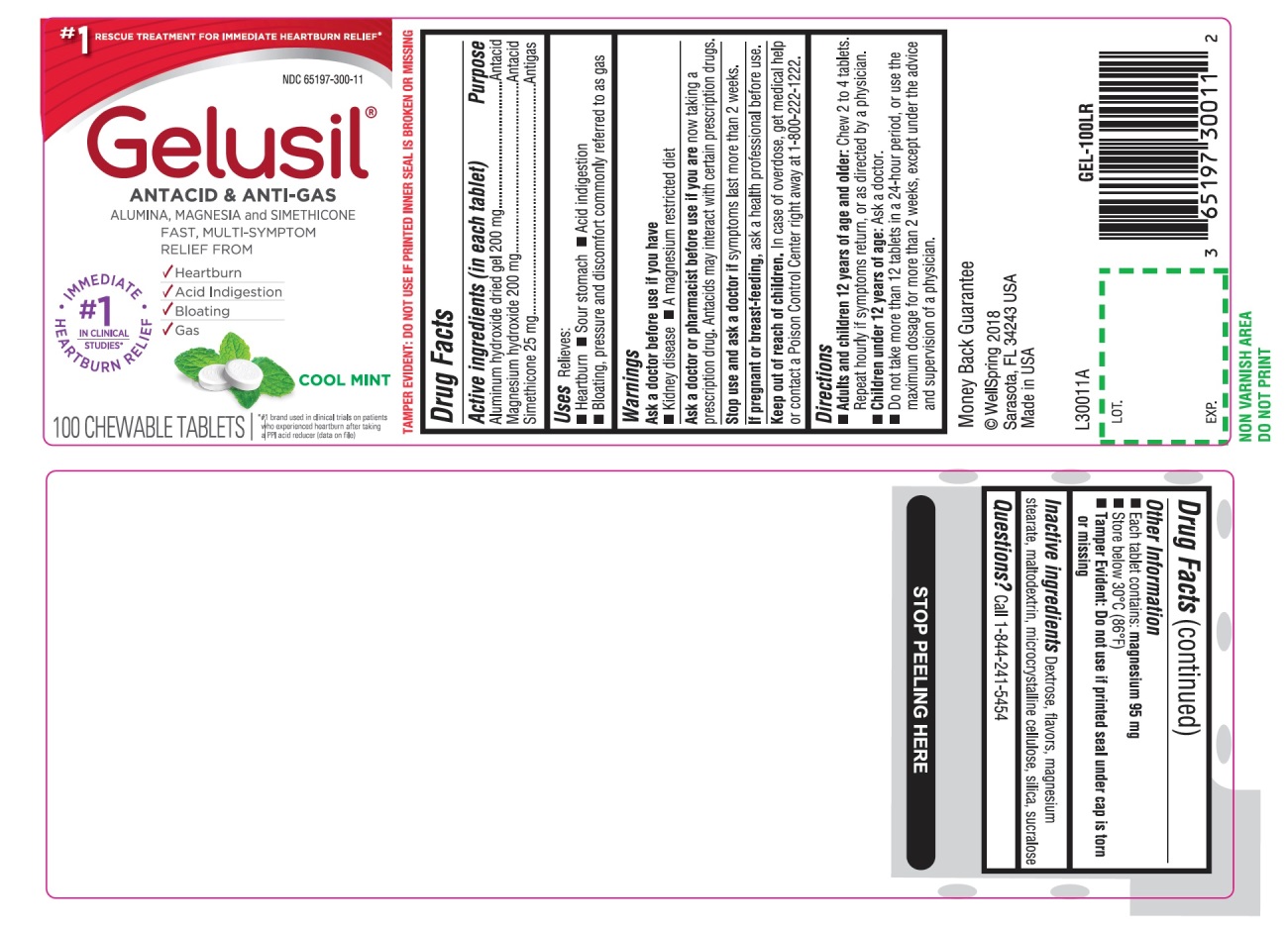
Active Ingredients (in each tablet)
Aluminum hydroxide dried gel 200 mg
Magnesium hydroxide 200 mg
Simethicone 25 mg
Purpose
Active ingredients (in each tablet) Purpose
Aluminum hydroxide dried gel 200 mg........................Antacid
Magnesium hydroxide 200 mg....................................Antacid
Simethicone 25 mg......................................................Antigas
Uses
Relieves:
- •
- Heartburn
- •
- Sour stomach
- •
- Acid indigestion
- •
- Bloating, pressure and discomfort commonly referred to as gas
Warnings
Ask a doctor or pharmacist before use if you are
now taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
- •
- Adults and children 12 years of age and older: Chew 2 to 4 tablets. Repeat hourly if symptoms return, or as directed by a physician
- •
- Children under 12 of age: ask a doctor
- •
- Do not take more than 12 tablets in a 24-hour period, or use the maximum dosage for more than 2 weeks, except under the advice and supervision of a physician
Other information
- •
- Each tablet contains: magnesium 95 mg
- •
- Store below 30°C (86°F)
- •
- Tamper Evident. Do not use if blister or printed Gelusil foil seal is broken
- •
- See end panel for lot number and expiration date