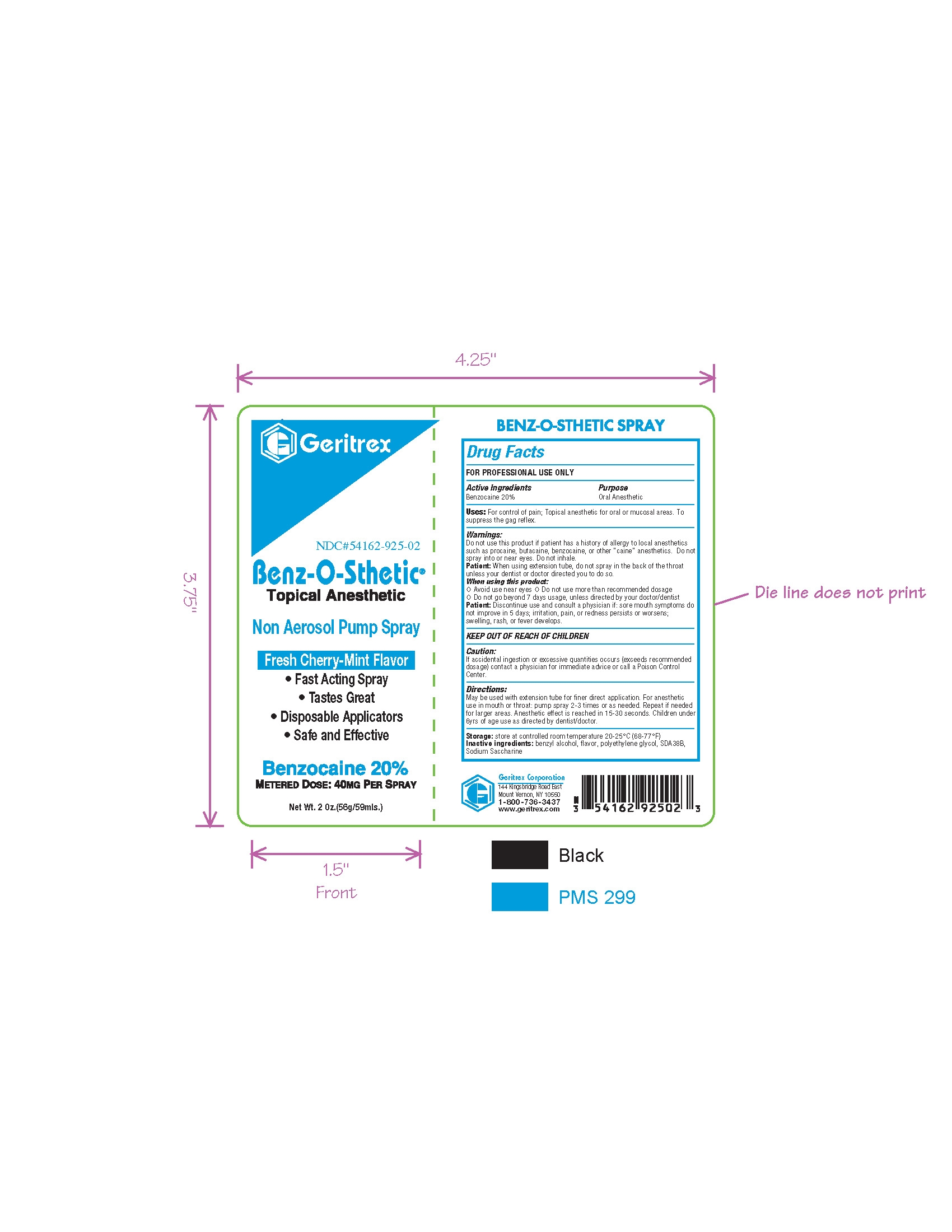
Directions
May be used with extension tube for finer direct application. For anestheticuse in mouth or throat: pump spray 2-3 times or as needed. Repeat if needed
for larger areas. Anesthetic effect is reached in 15-30 seconds.
Warnings
Do not use this product if patient has a history of allergy to local anesthetics
such as procaine, butacaine, benzocaine, or other “caine” anesthetics. Do not
spray into or near eyes. Do not inhale.
Patient: When using extension tube, do not spray in the back of the throat
unless your dentist or doctor directed you to do so.
When using this product:
Π Avoid use near eyes Π Do not use more than recommended dosage
Π Do not go beyond 7 days usage, unless directed by your doctor/dentist
Patient: Discontinue use and consult a physician if: sore mouth symptoms do
not improve in 5 days; irritation, pain, or redness persists or worsens;
swelling, rash, or fever develops.
 Enter section text here
Enter section text here