Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
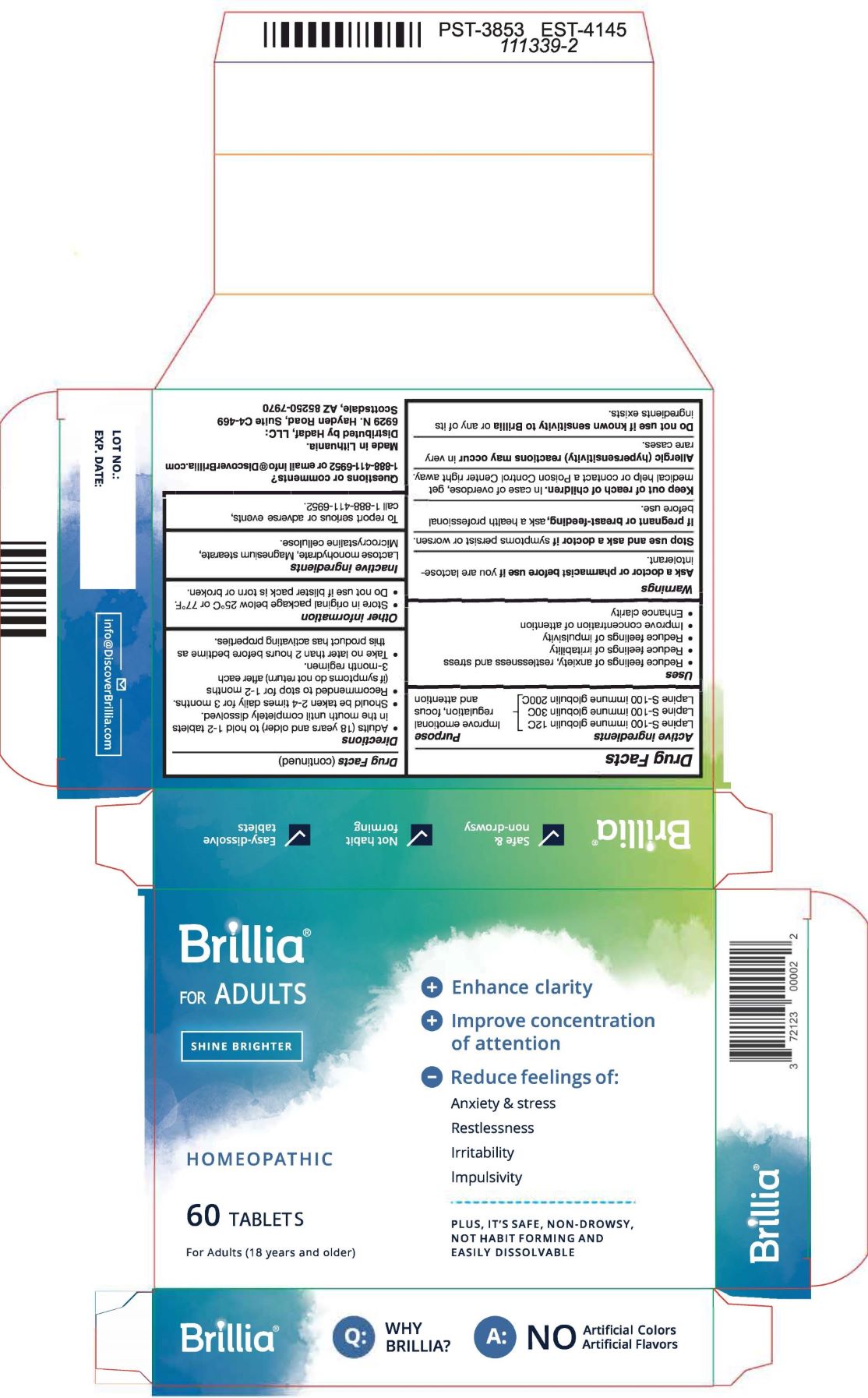
Uses
- reduce feelings of anxiety, restlessness, and stress
- reduce feelings of irritability
- reduce feelings of impulsivity
- improve concentration of attention
- enhance clarity
Directions
- Adults (18 years and older) to hold 1-2 tablets in the mouth until completely dissolved.
- Should be taken 2-4 times daily for 3 months
- Recommended to stop for 1-2 months (if symptoms do not return) after each 3 month regimen.
- Take no later than 2 hours before bedtime as this product has activating properties
Warnings
- Ask a doctor or pharmacist before use if you are lactose-intolerant
- Stop use and ask a doctor if symptoms persist or worsen
- If pregnant or breast-feeding, ask a health professional before use.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control right away.
- Allergic (hypersensitivity) reactions may occur in very rare cases.
- Do not use if known sensitivity to Brillia or any of its ingredients exists.