Active ingredient
Terbinafine hydrochloride 1%
Uses
- cures most athlete's foot (tinea pedis)
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- relieves itching, burning, cracking and scaling which accompany these conditions
Warnings
For external use only
Do not use
- on nails or scalp
- in or near the mouth or eyes
- for vaginal yeast infections
When using this product do not get into eyes. If eye contact occurs, rinse thoroughly with water.
Stop use and ask a doctor if too much irritation occurs or gets worse
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over:
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
-
for athlete's foot wear well-fitting, ventilated shoes.
Change shoes and socks at least once daily.
-
between the toes only:
apply twice a day (morning and night) for 1 week or as directed by a doctor
-
on the bottom or sides of the foot:
apply twice a day (morning and night) for 2 weeks or as directed by a doctor
-
for jock itch and ringworm:
apply once a day (morning or night) for 1 week or as directed by a doctor
- wash hands after each use
- children under 12 years: ask a doctor
| 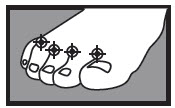 1 week between the toes 1 week between the toes 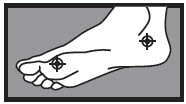 2 weeks on the bottom or sides of the foot 2 weeks on the bottom or sides of the foot |
Other information
TAMPER EVIDENT: DO NOT USE IF THE SEAL ON THE TUBE IS PUNCTURED OR NOT VISIBLE.
- store at controlled room temperature 20° to 25°C (68° to 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol, cetyl alcohol, cetyl palmitate, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, stearyl alcohol
Questions?
call 1-866-923-4914
Distributed by: Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
PRINCIPAL DISPLAY PANEL - 30 g Tube Carton
NDC 51672-2080-2
Full Prescription Strength
FOR ATHLETE'S FOOT
Terbinafine Hydrochloride
Cream 1%
Antifungal
NET WT 1 oz (30 g)
Taro Pharmaceuticals U.S.A., Inc.
 1 week between the toes
1 week between the toes  2 weeks on the bottom or sides of the foot
2 weeks on the bottom or sides of the foot 