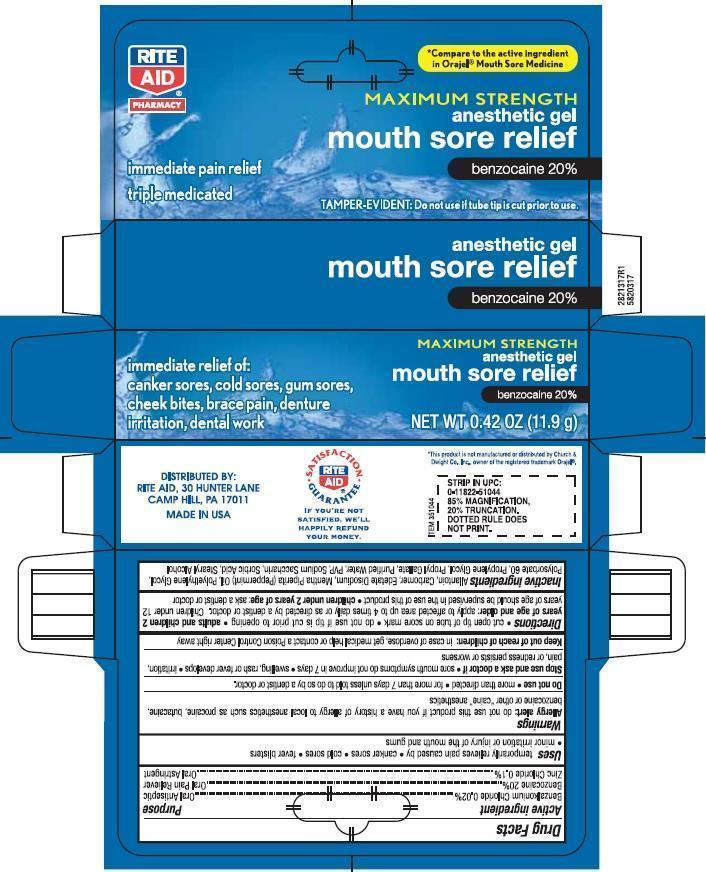
Active ingredient
Benzalkonium Chloride 0.02% .................................................... Oral Antiseptic
Benzocaine 7.5% ...................................................................... Oral Pain Reliever
Zinc Chloride 0.1% ................................................................... Oral Astringent
Uses
temporarily relieves pain caused by canker sores, cold sores, fever blisters, minor irritation or injury of the mouth and gums
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as
procaine, butacaine, benzocaine or other “caine” anesthetics
- Do not use more than directed for more than 7 days unless told to do so by a dentist or doctor.
- Stop use and ask a doctor if sore mouth symptoms do not get better in 7 days. swelling, rash or fever develops, irritation, pain or redness persists or worsens
Directions
cut open tip of tube on score mark. do not use if tip is cut prior to opening
Adults and children 2 years of age and older: apply to affected area up to 4 times daily or as directed by a doctor. Children under 12 years should be supervised in the use of this product.
Childern under 2 years of age: ask a dentist or doctor
Adults and children 2 years of age and older: apply to affected area up to 4 times daily or as directed by a doctor. Children under 12 years should be supervised in the use of this product.
Childern under 2 years of age: ask a dentist or doctor