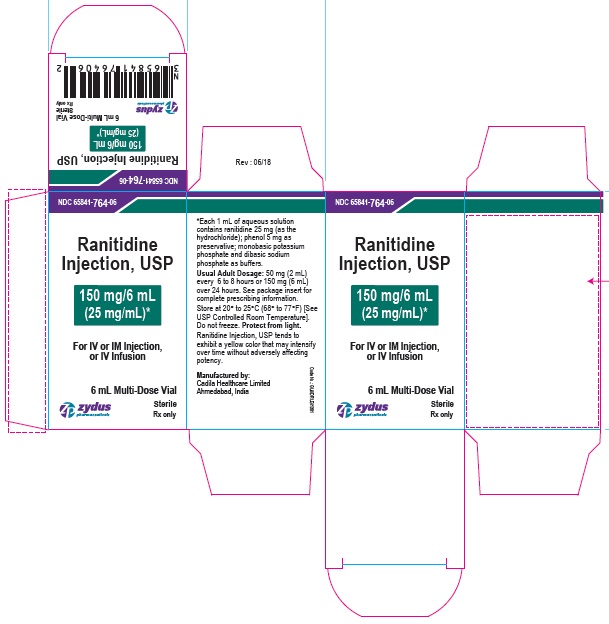
PRINCIPAL DISPLAY PANEL - 2 mL Vial Container Label
NDC 65841-763-02
Ranitidine Injection, USP
50 mg/2 mL (25 mg/mL)*
For IV or IM Injection, or IV Infusion
2 mL Single-Use Vial
Sterile
Rx only
Zydus Pharmaceuticals
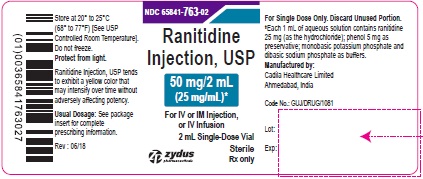
PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton Label
NDC 65841-763-02
Ranitidine Injection, USP
50 mg/2 mL (25 mg/mL)*
For IV or IM Injection, or IV Infusion
10 X 2 mL Single-Use Vials
Rx only
Sterile
Zydus Pharmaceuticals
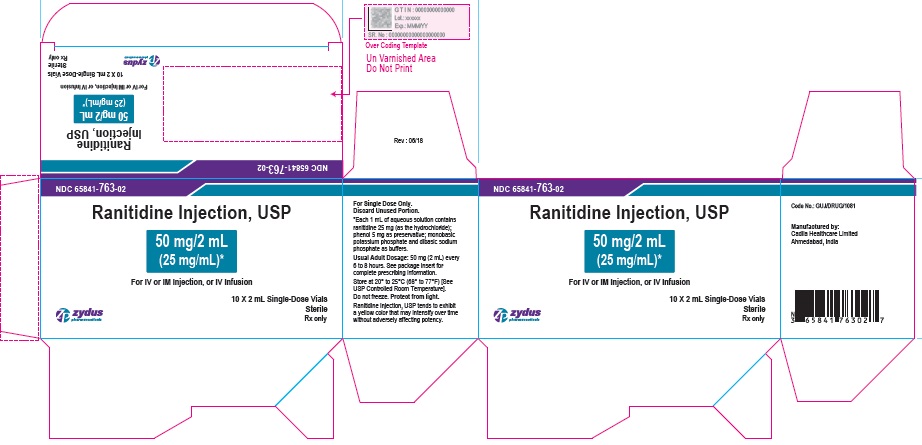
PRINCIPAL DISPLAY PANEL - 6 mL Vial Container Label
NDC 65841-764-06
Ranitidine Injection, USP
150 mg/6 mL (25 mg/mL)*
For IV or IM Injection, or IV Infusion
6 mL Multi-Dose Vial
Sterile
Rx only
Zydus Pharmaceuticals

PRINCIPAL DISPLAY PANEL - 6 mL Vial Carton Label
NDC 65841-764-06
Ranitidine Injection, USP
150 mg/6 mL (25 mg/mL)*
For IV or IM Injection, or IV Infusion
6 mL Multi-Dose Vial
Sterile
Rx only
Zydus Pharmaceuticals