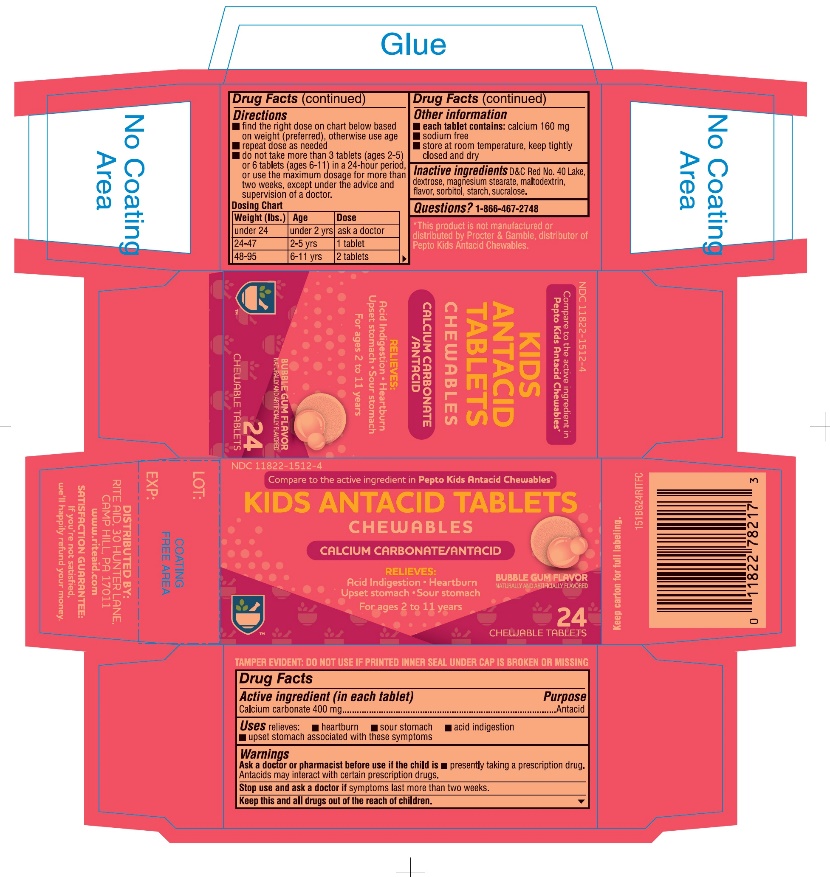
Uses
relieves:
- ▪
- heartburn
- ▪
- sour stomach
- ▪
- acid indigestion
- ▪
- upset stomach due to these symptoms or overindulgence in food and drink
Warnings
Directions
- ▪
- find the right dose on chart below based on weight (preferred), otherwise use age
- ▪
- repeat dose as needed
- ▪
- do not take more than 3 tablets (ages 2-5) or 6 tablets (ages 6-11) in a 24-hour period, or use the maximum dosage for more than two weeks, except under the advice and supervision of a doctor.
Dosing Chart
| Weight (lbs.) | Age | Dose |
|---|---|---|
|
under 24 |
under 2 yrs |
ask a doctor |
|
24-47 |
2-5 yrs |
1 tablet |
|
48-95 |
6-11 yrs |
2 tablets |
Other information
- •
- each tablet contains: calcium 160 mg
- •
- sodium free
- •
- store at room temperature, keep tightly closed and dry.
Inactive ingredients
D&C Red No. 40 Lake, dextrose, magnesium stearate, maltodextrin, flavor, sorbitol, starch, sucralose.
Package Label Principal Display Panel - 24 Tablet Carton
Compare to the active ingredient in Pepto Kids Antacid Chewables*
NDC 11822-1512-4
Relieves:
- •
- Acid Indigestion
- •
- Heartburn
- •
- Sour Stomach
- •
- Upset Stomach
24 Chewable Tablets
For ages 2 to 11 years
Bubble Gum Flavor
Naturally and Artificially Flavor