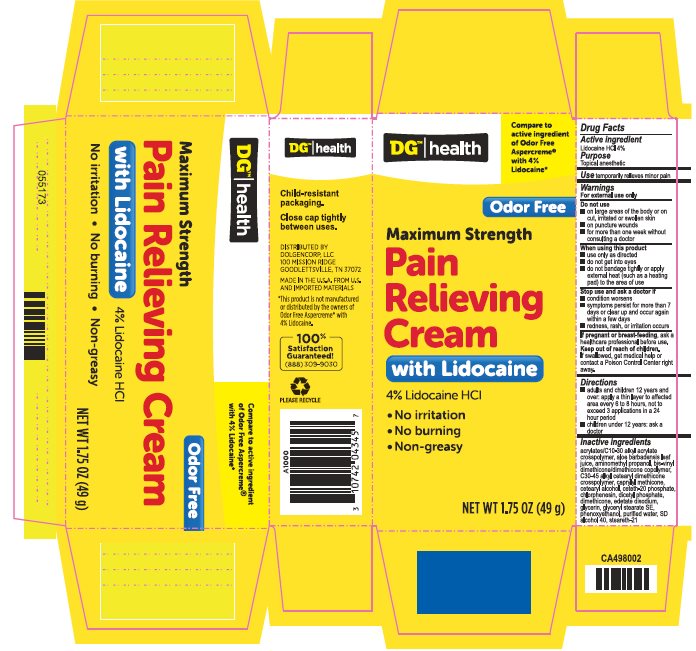
Warnings
For external use only
Do not use
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over: apply a thin layer to affected area every 6 to 8 hours, not to exceed 3 applications in a 24 hour period
- children under 12 years: ask a doctor
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, aminomethyl propanol, bis-vinyl dimethicone/dimethicone copolymer, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, chlorphenesin, dicetyl phosphate, dimethicone, edetate disodium, glycerin, glyceryl stearate SE, phenoxyethanol, purified water, SD alcohol 40, steareth-21