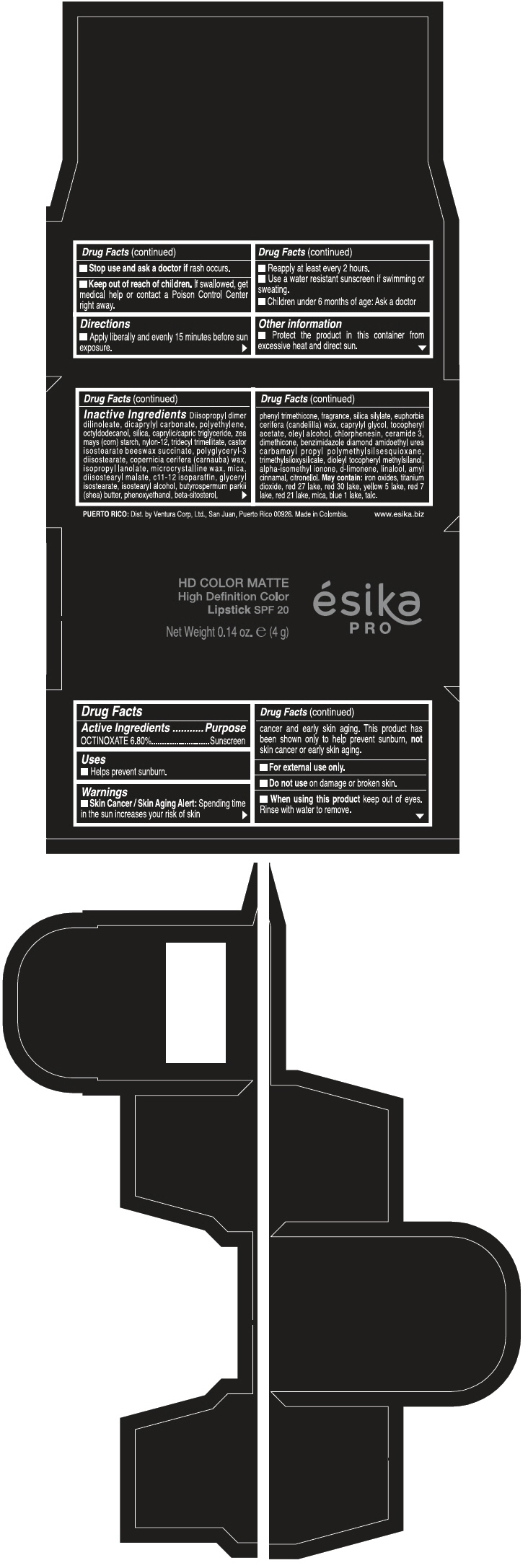
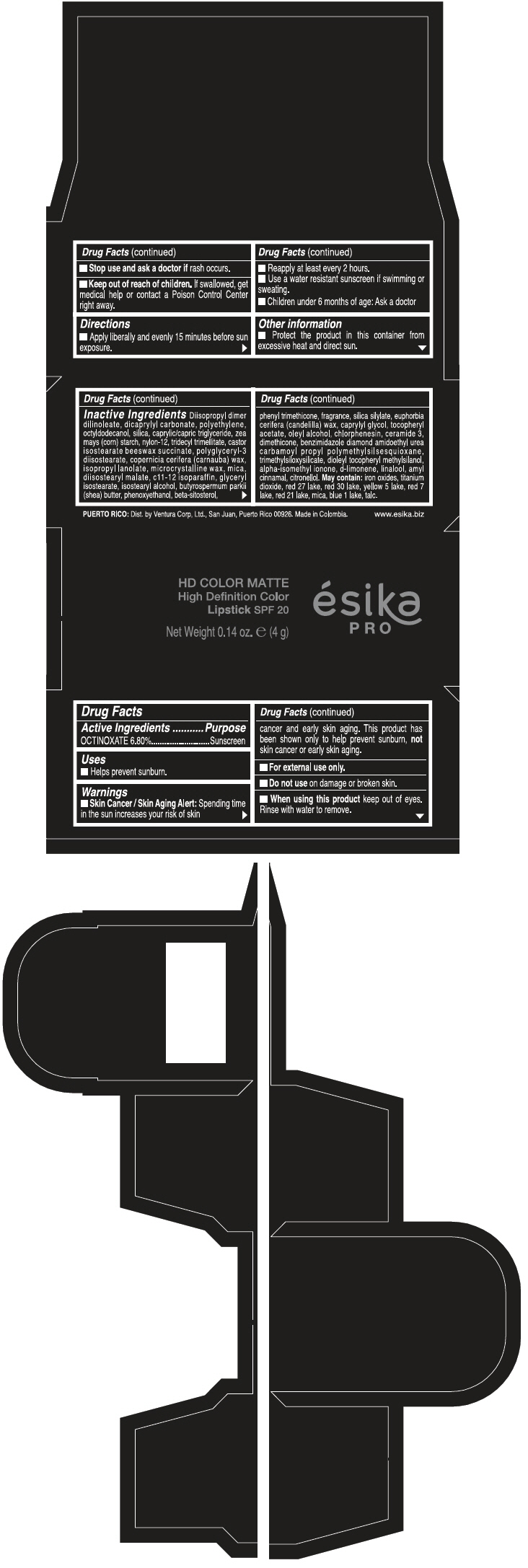
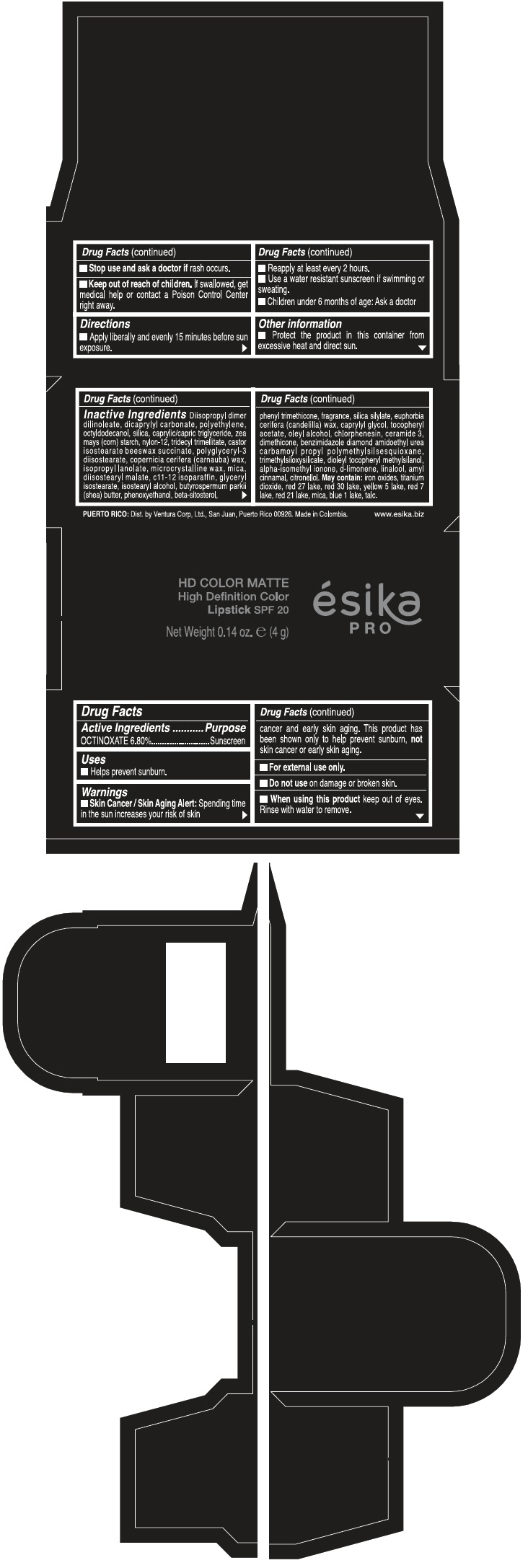
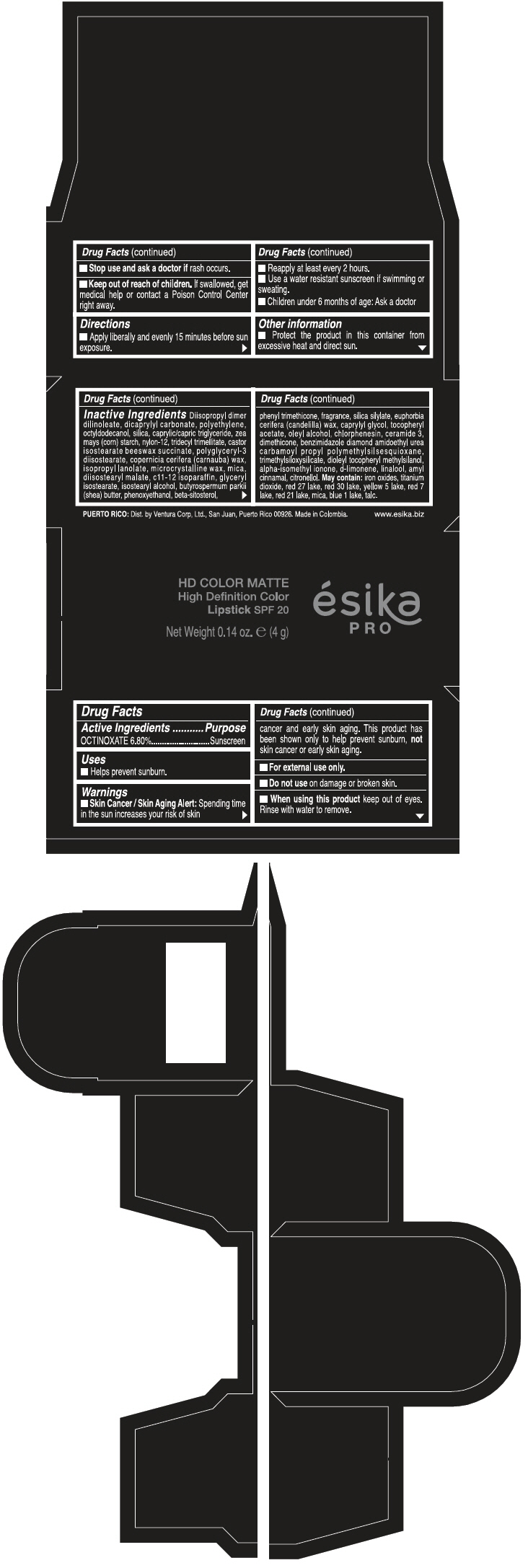
Warnings
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor
Inactive ingredients
DIISOPROPYL DIMER DILINOLEATE, DICAPRYLYL CARBONATE, POLYETHYLENE, OCTYLDODECANOL, SILICA, CAPRYLIC/CAPRIC TRIGLYCERIDE, ZEA MAYS (CORN) STARCH, NYLON-12, TRIDECYL TRIMELLITATE, CASTOR ISOSTEARATE BEESWAX SUCCINATE, POLYGLYCERYL-3 DIISOSTEARATE, COPERNICIA CERIFERA (CARNAUBA) WAX, ISOPROPYL LANOLATE, MICROCRYSTALLINE WAX, MICA, DIISOSTEARYL MALATE, C11-12 ISOPARAFFIN, GLYCERYL ISOSTEARATE, ISOSTEARYL ALCOHOL, BUTYROSPERMUM PARKII (SHEA) BUTTER, PHENOXYETHANOL, BETA-SITOSTEROL, PHENYL TRIMETHICONE, FRAGRANCE, SILICA SILYLATE, EUPHORBIA CERIFERA (CANDELILLA) WAX, CAPRYLYL GLYCOL, TOCOPHERYL ACETATE, OLEYL ALCOHOL, CHLORPHENESIN, CERAMIDE 3, DIMETHICONE, BENZIMIDAZOLE DIAMOND AMIDOETHYL UREA CARBAMOYL PROPYL POLYMETHYLSILSESQUIOXANE, TRIMETHYLSILOXYSILICATE, DIOLEYL TOCOPHERYL METHYLSILANOL, ALPHA-ISOMETHYL IONONE, d-LIMONENE, LINALOOL, AMYL CINNAMAL, CITRONELLOL. MAY CONTAIN: IRON OXIDES, TITANIUM DIOXIDE, RED 27 LAKE, RED 30 LAKE, YELLOW 5 LAKE, RED 7 LAKE, RED 21 LAKE, MICA, BLUE 1 LAKE, TALC.
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FUCSIA GALA MATE - PURPLE
HD COLOR MATTE
High Definition Color
Lipstick SPF 20
ésika
PRO
Net Weight 0.14 oz. e (4 g)
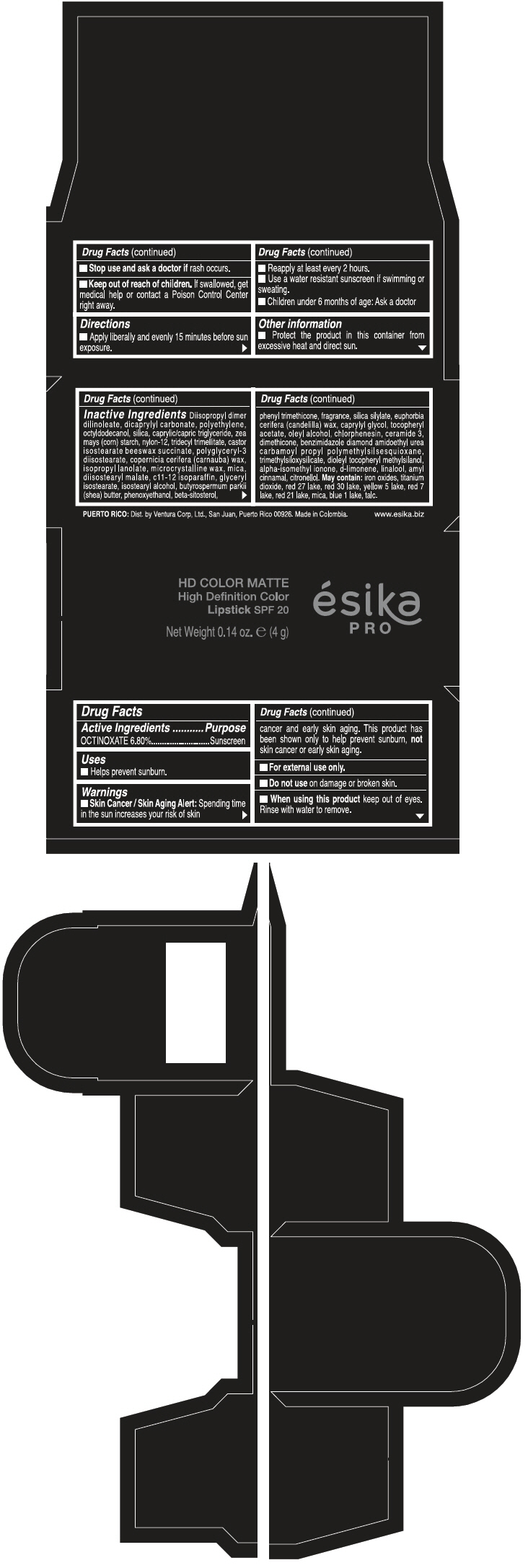
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FRAMBUESA GLAM MATE - RED
HD COLOR MATTE
High Definition Color
Lipstick SPF 20
ésika
PRO
Net Weight 0.14 oz. e (4 g)
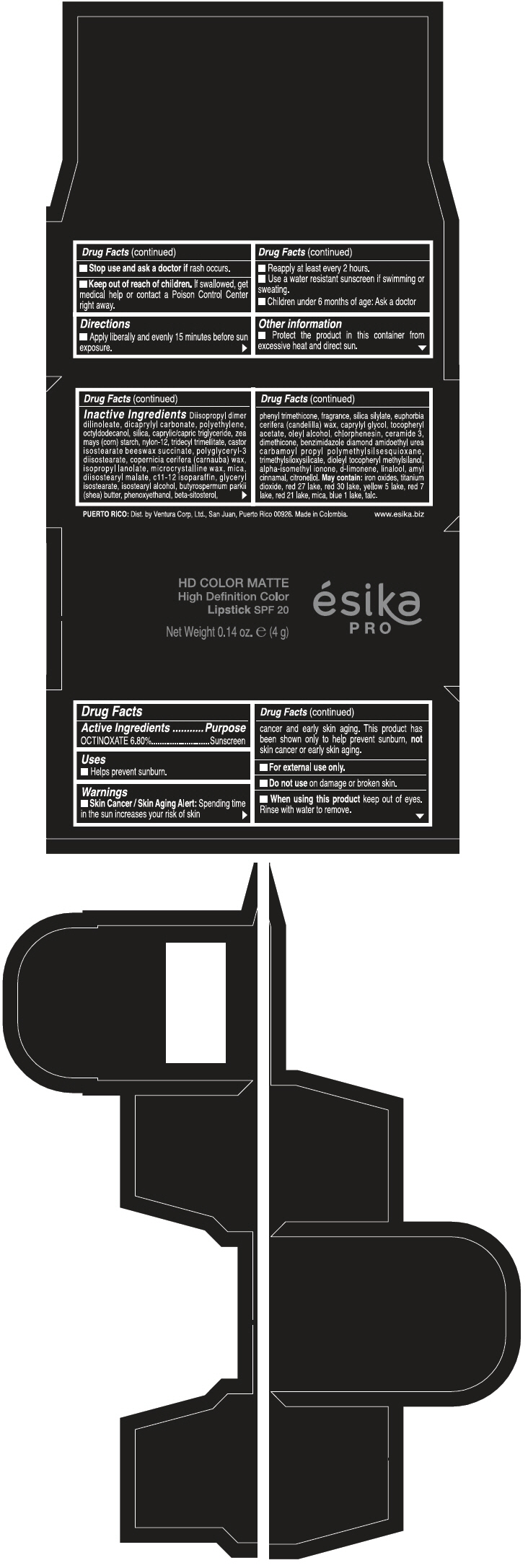
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROSA DESEO MATE - PINK
HD COLOR MATTE
High Definition Color
Lipstick SPF 20
ésika
PRO
Net Weight 0.14 oz. e (4 g)
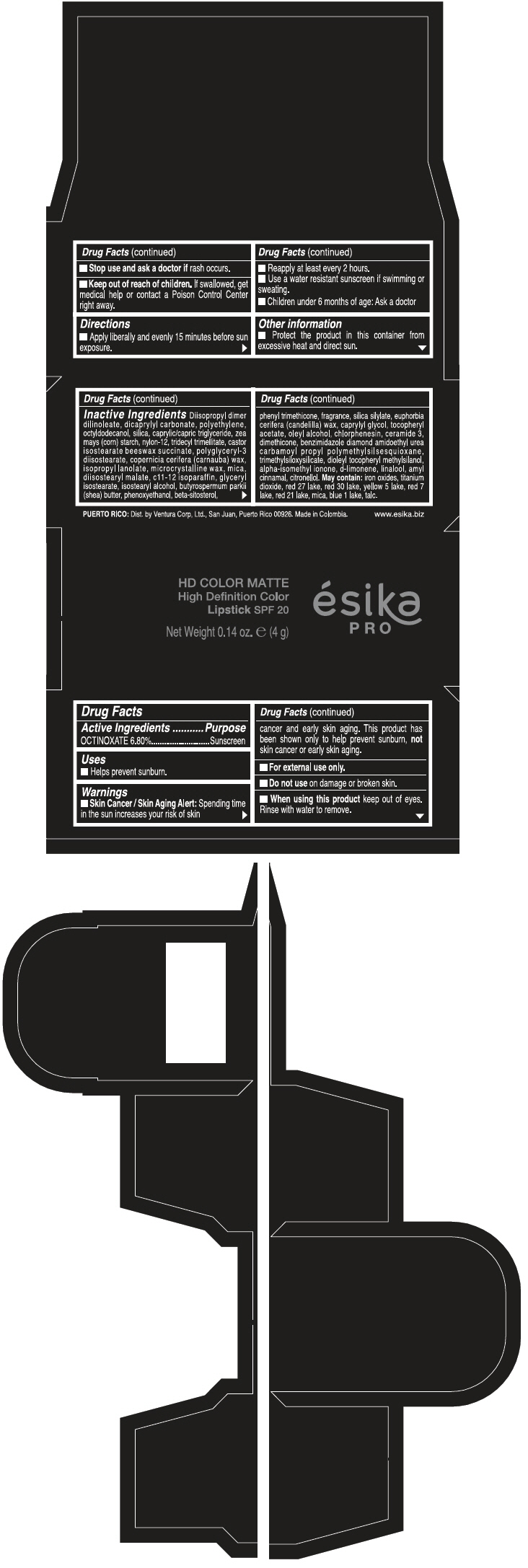
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FUCSIA PINK MATE - PURPLE
HD COLOR MATTE
High Definition Color
Lipstick SPF 20
ésika
PRO
Net Weight 0.14 oz. e (4 g)
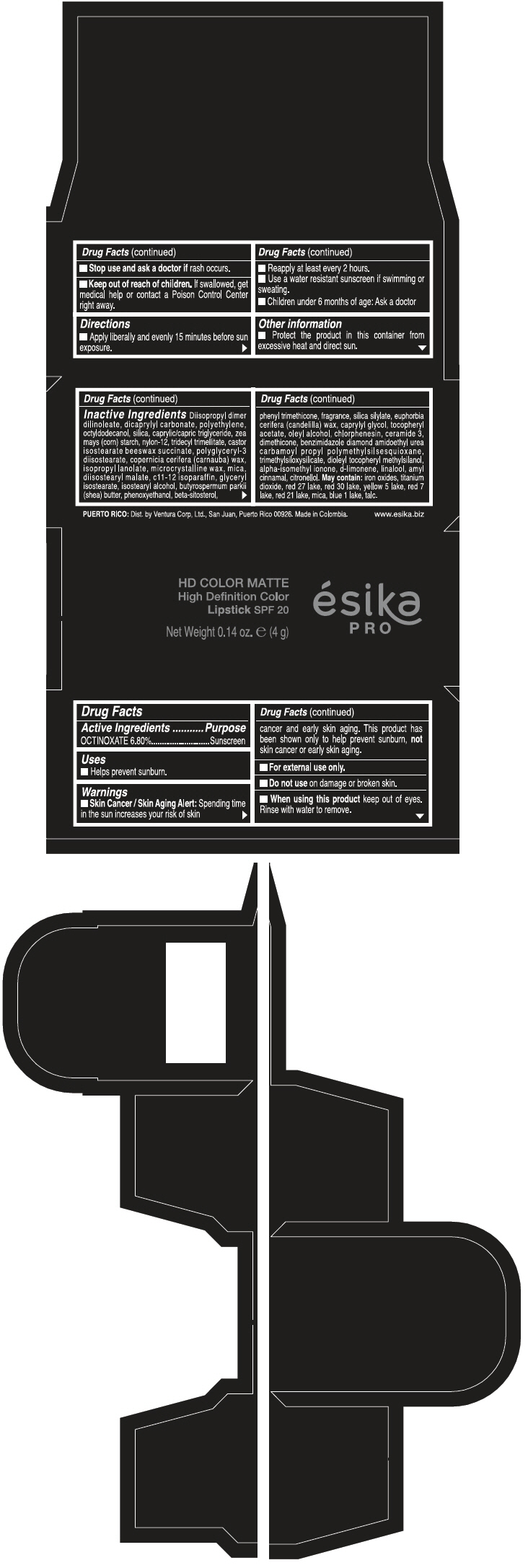
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROJO PASION MATE - RED
HD COLOR MATTE
High Definition Color
Lipstick SPF 20
ésika
PRO
Net Weight 0.14 oz. e (4 g)
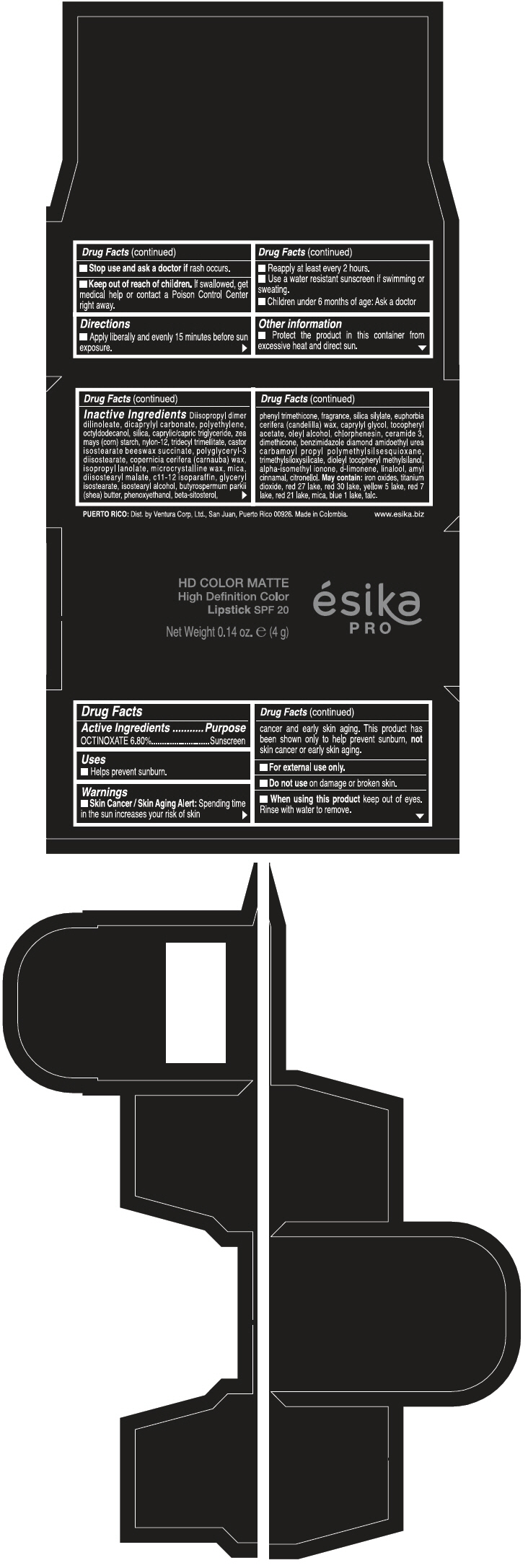
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - PIMIENTA CALIENTE MATE - BROWN
HD COLOR MATTE
High Definition Color
Lipstick SPF 20
ésika
PRO
Net Weight 0.14 oz. e (4 g)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FRESA PROFUNDO MATE - RED
HD COLOR MATTE
High Definition Color
Lipstick SPF 20
ésika
PRO
Net Weight 0.14 oz. e (4 g)