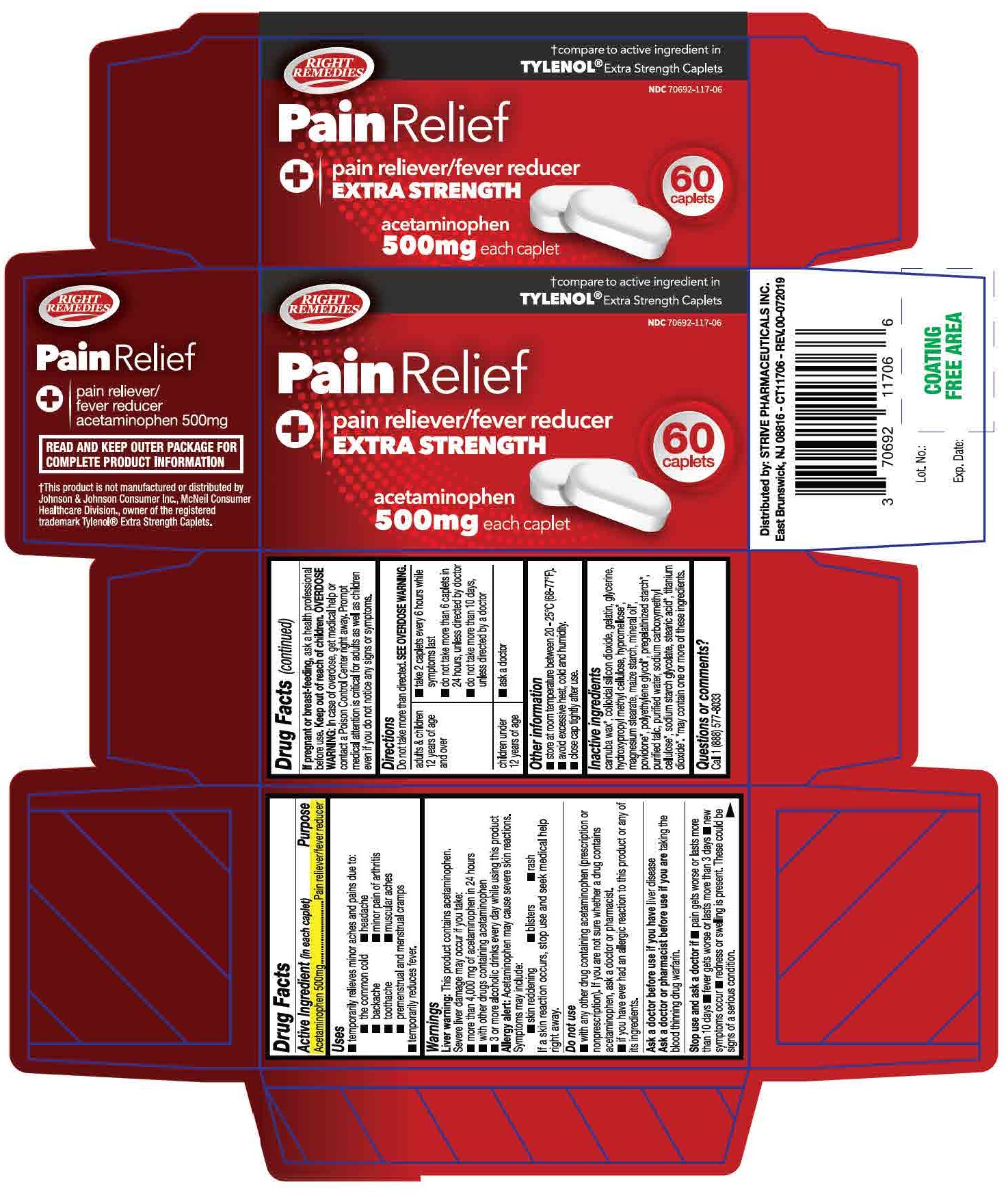
colloidal silicon dioxide, gelatin, glycerin, hydroxypropyl methyl cellulose, maize starch, magnesium stearate, purified talc, sodium starch glycolate
- take with a full glass of water
- do not take more than directed
| adults and children 12 years of age and over |
|
| children under 12 years of age | ask a doctor |
*temporarily relieves minor aches and pains due to:
- the common cold
- headache
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
*temporarily reduces fever
Liver warning:
This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4000mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert:
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
if a skin reaction occurs, stop use and seek medical help right away
Do not use
- with any other drug containing acetaminophen (prescription or non prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor before use if you have
- liver disease
Ask a doctor or a pharmacist before use if
you are taking the blood thinning drug warfarin
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious comdition
If pregnant or breast-feeding,
ask a health professional before use
Keep out of reach of children
Overdose warning: In case of accidental overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.