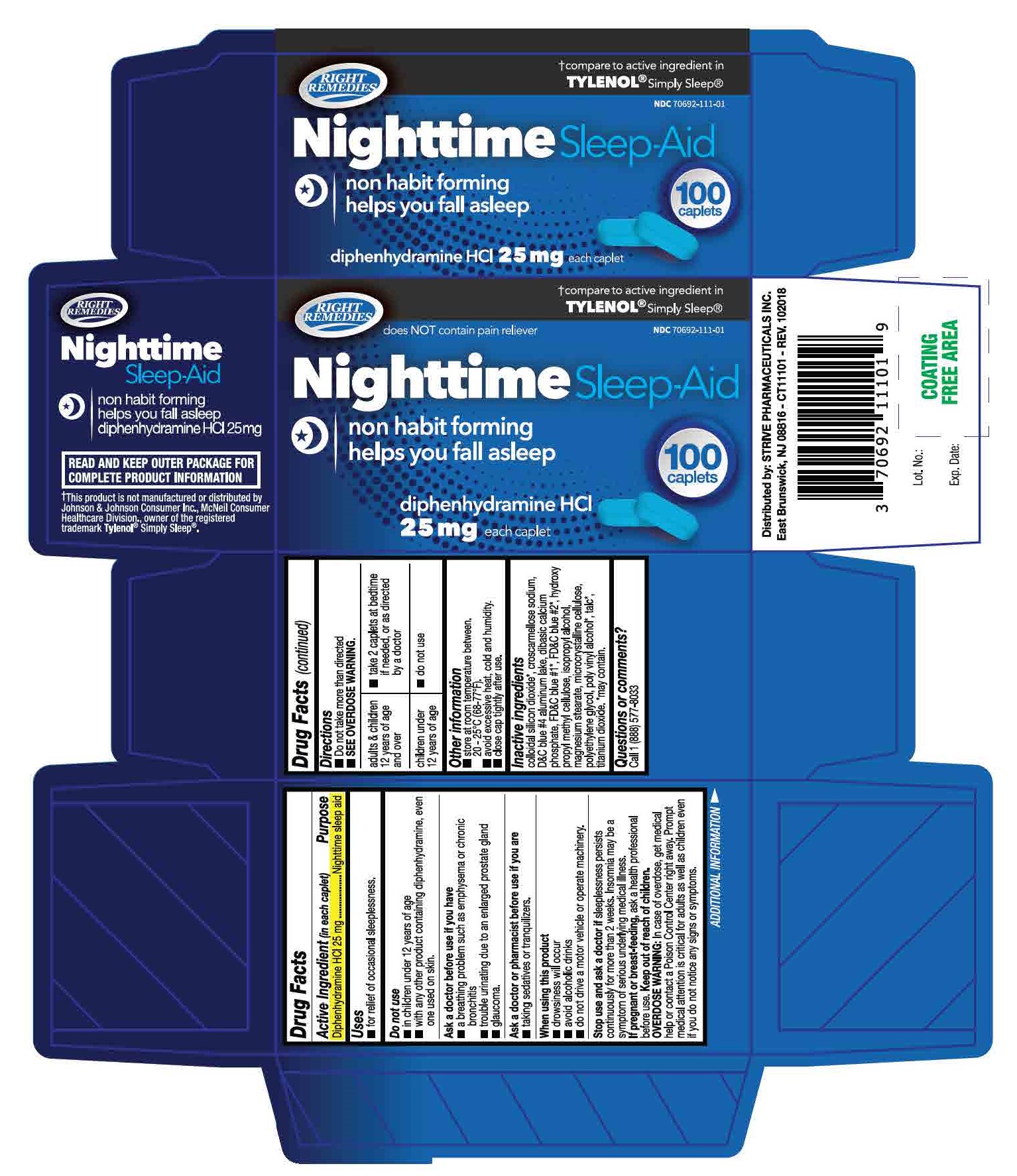
croscarmellose sodium, D&C blue #4 aluminium lake, dibasic calcium phosphate, hydroxy propyl methyl cellulose, isopropyl alcohol, magnesium stearate, microcrystalline cellulose, polyethyl;ene glycol, talc, titanium dioxide
- take with a full glass of water
- do not take more than directed
| adults and children 12 years of age and over | 2 caplets at bedtime if needed, or as directed by a doctor |
| children under 12 years of age | do not use |
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostrate gland
Ask a doctor or a pharmacist before use if
you are taking sedatives or tranquilzers
When using this product
- avoid alcoholic drinks
- drowsiness will occur
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness
If pregnant or breast-feeding,
ask a health professional before use
Keep out of reach of children
Overdose warning: In case of accidental overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Keep out of reach of children
Overdose Warning: In case of accidental overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.