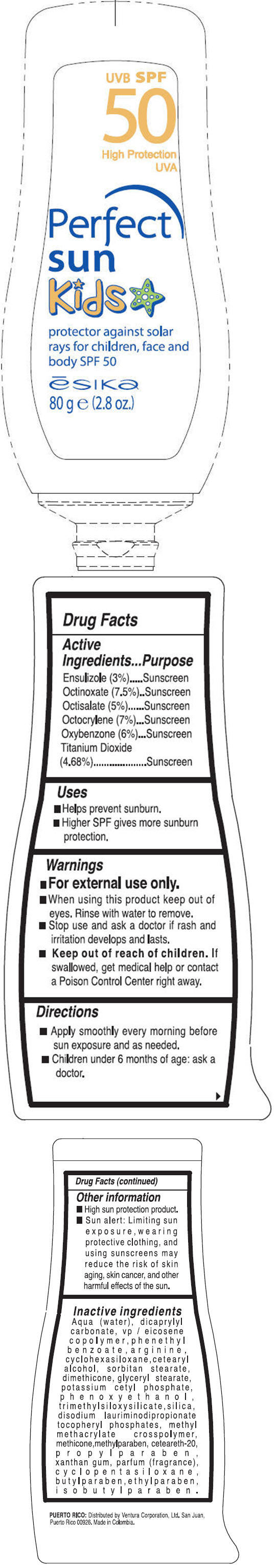
Active Ingredients
Ensulizole (3%), Octinoxate (7.5%), Octisalate (5%), Octocrylene (7%), Oxybenzone (6%),Titanium Dioxide (4.68%)
Directions
- Apply smoothly every morning before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
Other information
- High sun protection product.
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
Aqua (water), dicaprylyl carbonate, vp/eicosene copolymer, phenethyl benzoate, arginine, cyclohexasiloxane, cetearyl alcohol, sorbitan stearate, dimethicone, glyceryl stearate, potassium cetyl phosphate, phenoxyethanol, trimethylsiloxysilicate, silica, disodium lauriminodipropionate tocopheryl phosphates, methyl methacrylate crosspolymer, methicone, methylparaben, ceteareth-20, propylparaben, xanthan gum, parfum (fragrance), cyclopentasiloxane, butylparaben, ethylparaben, isobutylparaben.