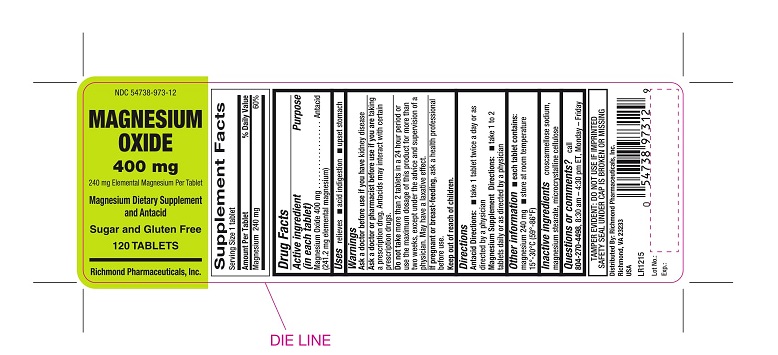
MAGNESIUM OXIDE- magnesium oxide tablet
Richmond Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT (In each tablet)
Magnesium Oxide 400mg (241.2mg elemental magnesium)
USES
Relieves: ■ acid indigestion ■ upset stomach
WARNINGS
Ask a doctor if you have:
kidney disease
Ask a doctor or pharmacist before use if you are:
taking a prescription drug. Antacids may interact with certain prescription drugs.
Do not take
more than 2 tablets in a 24 hour period or use the maximum dosage of this product for more than two weeks, except under the advise and supervision of a physician. May have a laxative effect.
If pregnant or breast-feeding,
ask a health professional before use.
Keep Out of Reach of Children.
DIRECTIONS
Antacid Directions: ■ take 1 tablet twice a day or as directed by a physician
Magnesium Supplement Directions: ■ take 1 to 2 tablets daily or as directed by a physician
OTHER INFORMATION
■ store at room temperature 59°-86° F (15°-30°C) ■ do not use if imprinted safety seal under cap is broken or missing
■ Magnesium content per tablet: 253 mg
INACTIVE INGREDIENTS
croscarmellose sodium, microcrystalline cellulose, magnesium stearate
Questions or Comments
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
call 804-270-4498, 8.30 am-4.30 pm ET, Monday - Friday
PRINCIPAL DISPLAY PANEL
NDC 54738-973-12- 120 tablets
Richmond Pharmaceuticals, Inc.
