FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
CLOROTEKAL® (chloroprocaine hydrochloride) is indicated for intrathecal injection for the production of subarachnoid block (spinal anesthesia) in adults undergoing surgical procedures. Indicated procedures include those suitable for CLOROTEKAL®'s short duration of action.
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
CLOROTEKAL® must only be administered by clinicians with the necessary knowledge and experience in the intrathecal anesthesia administration. The equipment, drugs, and personnel capable of dealing with an emergency, e.g. maintaining the patency of the airways and administering oxygen, must be immediately available, because in rare cases severe reactions, sometimes with a fatal outcome, have been reported after using local anesthetics, even in the absence of individual hypersensitivity in the patient's case history.
- Visually inspect parental drug products for particulate matter and discoloration prior to administration, whenever solution and container permit.
- CLOROTEKAL® is intended for single-use only. Discard any unused portion in an appropriate manner.
- CLOROTEKAL® must be drawn up with a filter needle. Use CLOROTEKAL® immediately after first opening. Protect from light. Do not freeze, heat, or autoclave [see How Supplied/Storage and Handling (16)].
- In the absence of safety studies and compatibility studies, this product must not be mixed or diluted with other products. In addition, this product should not be substituted with a different chloroprocaine product.
- The safety of CLOROTEKAL® administration via continuous spinal catheters has not been established and administration by this route is not recommended.
2.2 Administration
Not for epidural administration.
Monitor vital signs during dural puncture and provide oxygen via face mask or nasal cannula. Slowly inject the entire dose, while monitoring the patient's vital signs.
In general, the following points should be taken into consideration:
- A free flow of cerebrospinal fluid during the performance of spinal anesthesia is indicative of entry into the subarachnoid space.
- To avoid intravascular injection, aspiration should be performed before the anesthetic solution is injected. The needle must be repositioned until no blood return can be elicited. However, the absence of blood in the syringe does not guarantee that intravascular injection has been avoided.
- Do not puncture the skin if there are signs of infection or inflammation.
- The patient should have IV fluids running via an indwelling catheter to assure functioning intravenous access.
2.3 Recommended Dosing
The extent and degree of spinal anesthesia depend upon several factors including dosage, specific gravity of the anesthetic solution, volume of solution used, force of injection, level of puncture, and position of the patient during and immediately after injection.
To obtain an effective block to the T 10 level with one single administration in an adult of average height and weight (approximately 70 kg), the recommended dose is 50 mg.
Doses above 50 mg have not been adequately tested for efficacy and safety.
3 DOSAGE FORMS AND STRENGTHS
CLOROTEKAL® is supplied as a single-dose sterile, clear, colorless solution in a Type I (USP) glass ampule that provides 50 mg of chloroprocaine hydrochloride in 5 mL aqueous solution (concentration: 10 mg/mL) equivalent to 44.05 mg/5 mL (8.81 mg/mL) chloroprocaine.
4 CONTRAINDICATIONS
- CLOROTEKAL® is contraindicated in patients with a known hypersensitivity to the active substance, medicinal products of the PABA (para-aminobenzoic acid) ester group, other ester-type local anesthetics or to any of the excipients [see Risk of Hypersensitivity Reactions (5.4)]
- General and specific contraindications to spinal anesthesia regardless of the local anesthetic used, should be taken into account (e.g., decompensated cardiac insufficiency, hypovolemic shock, coagulopathy)
- Intravenous regional anesthesia (the anesthetic agent is introduced into the limb and allowed to set in while tourniquets retain the agent within the desired area)
- Serious problems with cardiac conduction
- Local infection at the site of proposed lumbar puncture
- Septicemia
5 WARNINGS AND PRECAUTIONS
5.1 Risks with Neuraxial Administration
Local anesthetics should only be administered by clinicians who are well versed in diagnosis and management of dose-related toxicity and other acute emergencies which might arise from the block to be employed, and then only after insuring the immediate availability of oxygen, other resuscitative drugs, cardiopulmonary resuscitative equipment, and the personnel resources needed for proper management of toxic reactions and related emergencies [see Adverse Reaction (6) and Overdosage (10)]. Delay in proper management of dose-related toxicity, underventilation from any cause and/or altered sensitivity may lead to the development of acidosis, cardiac arrest, and, possibly, death.
The clinician should take the appropriate measures to avoid an intravascular injection [see Administration (2.2)]. In addition, it is essential for the clinician to know how to recognize and treat undesirable effects, systemic toxicity and other complications. If signs of acute systemic toxicity or total spinal block are observed, the injection of the local anesthetic must be stopped immediately [see Overdosage (10)].
5.2 Cardiovascular System Reactions
Hypotension and bradycardia are well known side effects of all local anesthetics [see Adverse Reaction (6) and Overdosage (10)].
A serious, undesirable effect of spinal anesthesia is high or total spinal block, with consequent cardiovascular and respiratory depression. Cardiovascular depression is induced by an extended block of the sympathetic nervous system, which may induce severe hypotension and bradycardia to the point of cardiac arrest. Respiratory depression is induced by the block of the respiratory musculature and the diaphragm. Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be accomplished after CLOROTEKAL® injection.
Patients over 65 years, particularly those with hypertension, may be at increased risk for experiencing the hypotensive effects of CLOROTEKAL®. Blood pressure should, therefore, be carefully monitored after CLOROTEKAL® injection. Hypotension may be controlled by vasoconstrictors in dosages depending on the severity of hypotension and response of treatment.
5.3 Central Nervous System Reactions
Neurological damage may occur after spinal anesthesia, manifesting as paresthesia, loss of sensitivity, motor weakness, paralysis, cauda equina syndrome. Occasionally these symptoms persist and can be permanent. Carefully evaluate patients with underlying neuromuscular disorders and consider the risk-benefit ratio prior to treatment.
Carefully and constantly monitor cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness after local anesthetic injection. Restlessness, headache, anxiety, incoherent speech problems, lightheadedness, paresthesia, numbness and tingling of the mouth and lips, hearing problems, tinnitus, dizziness, blurred vision, convulsions, loss of consciousness tremors, depression, or drowsiness may be early warning signs of central nervous system toxicity [see Adverse Reactions (6.2) and Overdosage (10)].
5.4 Risk of Hypersensitivity Reactions
CLOROTEKAL® is contraindicated in patients hypersensitive to drugs of the PABA ester group. Allergic type reactions may occur as a result of sensitivity to the local anesthetic or to other formulation ingredients. These reactions are characterized by signs such as urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), tachycardia, sneezing, nausea, vomiting, dizziness, syncope, excessive sweating, elevated temperature, and possibly, anaphylaxis type symptomatology (including severe hypotension). Cross sensitivity among members of the ester-type local anesthetic group has been reported. The usefulness of screening for sensitivity has not been definitely established [see Adverse Reactions (6.2)].
5.5 Risk of Chondrolysis in Patients Receiving Intraarticular Injections
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain, stiffness, and loss of motion, can be variable, but may begin as early as the 2nd month after surgery. Currently, there is no effective treatment for chondrolysis. Patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
5.6 Conditions Requiring Special Attention
Some patients require special attention in order to reduce the risk of serious undesirable effects, even when locoregional anesthesia constitutes the optimum choice for the surgical intervention:
- Patients with total or partial heart block, since local anesthetics can suppress myocardial conduction
- Patients with high grade cardiac decompensation
- Patients with advanced liver or kidney damage [see Use in Specific Populations (8.6)]
- Elderly patients and patients in poor general condition [see Use in Specific Populations (8.5) and Clinical Pharmacology (12.3)]
- Patients with genetic deficiency of plasma cholinesterase [see Adverse Reactions (6) and Clinical Pharmacology (12.3)]
- Patients taking anticoagulants or with congenital or acquired bleeding disorder
- Patients with severe anemia
Because ester-type local anesthetics are hydrolyzed by plasma cholinesterase produced by the liver, use CLOROTEKAL® cautiously in patients with advanced hepatic disease [see Use in Specific Populations (8.6)].
Local anesthetics should also be used with caution in patients with impaired cardiovascular function since they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
6 ADVERSE REACTIONS
The following serious adverse reactions are described, or described in greater detail, in other sections:
- Cardiovascular System Reactions [see Warnings and Precautions (5.2)]
- Central Nervous System Reactions [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
During clinical investigations, a total of 111 patients undergoing various surgical procedures received CLOROTEKAL®. Patients were administered a dose ranging from 30 to 50 mg of CLOROTEKAL®.
Taking into consideration data for 50 mg dose only, the most common adverse reaction in these studies, (incidence greater than or equal to 10%) following CLOROTEKAL® administration was procedural pain.
The common adverse reactions (incidence greater than or equal to 2% to less than 10%) following CLOROTEKAL® administration were injection site pain and hypotension.
The less common/rare adverse reactions (incidence less than 2%) following CLOROTEKAL® administration were anesthetic complication, nausea, headache and hyperglycemia.
Adverse Reactions Reported in Controlled Trials
All Treatment Emergent Adverse Reactions in clinical studies comparing CLOROTEKAL® (dose 50 mg) to Bupivacaine 0.5% (10 mg) are shown in Table 1.
| System Organ Class Preferred Term | Chloroprocaine 10 mg/mL (50 mg) N = 81 n (%) | Bupivacaine 0.5% (10 mg) N=64 n (%) |
|---|---|---|
| Note: Subjects are summarized according to the product they actually received. The denominator for calculating the proportions is the number of subjects in each treatment group and overall. TEAE = Treatment-Emergent Adverse Event (both related and non-related). |
||
| Subjects with Any TEAE | 17 (21.0) | 3 (4.7) |
| Injury, Poisoning and Procedural Complications | 13 (16.0) | 0 (0.0) |
| Procedural Pain | 13 (16.0) | 0 (0.0) |
| General Disorders and Administration Site Conditions | 3 (3.7) | 2 (3.1) |
| Injection Site Pain | 3 (3.7) | 2 (3.1) |
| Vascular Disorders | 4 (4.9) | 1 (1.6) |
| Hypotension | 4 (4.9) | 1 (1.6) |
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval of use of CLOROTEKAL® outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure (Table 2).
| System Organ Class | Adverse Reactions |
|---|---|
| Immune System Disorders | Hypersensivity (including urticaria, pruritus, erythema multiforme, angioedema with possible airway obstruction), anaphylaxis |
| Injury, Poisoning and Procedural Complications | Urinary retention postoperative, delayed recovery from anesthesia, anesthetic complication |
| General Disorders and Administration Site Conditions | Feeling hot, malaise |
| Musculoskeletal and Connective Tissue Disorders | Back pain, groin pain, pain in extremity |
| Nervous System Disorders
| Restlessness, paresthesia, dizziness, tremor, seizure, oral paresthesia, oral hypoesthesia, hearing disability, visual disorders, blurred vision, tinnitus, speech disorders, loss of consciousness, peripheral neuropathy, somnolence, unintentional total spinal block, urinary and anal incontinence, perineal disorder and sexual dysfunction, arachnoiditis, akathisia, presyncope, burning sensation, spinal cord injury, cauda equina syndrome, hypoesthesia, dysesthesia, motor dysfunction, myoclonus, phantom pain, headache |
| Eye Disorders | Diplopia, photophobia |
| Cardiac Disorders
| Bradycardia, tachycardia, arrhythmia, myocardial depression, cardiac arrest |
| Psychiatric Disorders | Anxiety |
| Vascular Disorders | Hypertension, hypotension |
| Respiratory, Thoracic and Mediastinal Disorders | Respiratory depression, dyspnea, respiratory arrest |
| Gastrointestinal Disorders | Nausea, vomiting |
7 DRUG INTERACTIONS
Concurrent administration of vasopressor drugs (for the treatment of hypotension related to obstetric blocks) and ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.
The para-aminobenzoic acid metabolite of chloroprocaine inhibits the action of sulfonamides. Therefore, avoid use in any condition in which a sulfonamide drug is being employed [see Clinical Pharmacology (12.3)].
No studies have been performed on interactions between chloroprocaine and class III antiarrhythmics (e.g., amiodarone). Carefully monitor these patients for cardiovascular effects.
The combination of various local anesthetics may result in additive effects affecting the cardiovascular system and the central nervous system. Monitor these patients for signs and symptoms of local anesthetic toxicity.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data with chloroprocaine use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. There are no animal reproduction studies for chloroprocaine. There are risks to the mother and the fetus associated with use of chloroprocaine during labor and delivery (see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Labor or delivery
Local anesthetics rapidly cross the placenta, and when used for epidural, paracervical, pudendal or caudal block anesthesia, can cause varying degrees of maternal, fetal and neonatal toxicity [see Clinical Pharmacology (12.3)]. The incidence and degree of toxicity depend upon the procedure performed, the type and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus and neonate involve alterations of the central nervous system, peripheral vascular tone and cardiac function.
Spinal anesthesia may alter the forces of parturition through changes in uterine contractility or maternal expulsive efforts. Spinal anesthesia has also been reported to prolong the second stage of labor by removing the parturient's reflex urge to bear down or by interfering with motor function. The use of obstetrical anesthesia may increase the need for forceps assistance.
The use of some local anesthetic drug products during labor and delivery may be followed by diminished muscle strength and tone for the first day or two of life.
Maternal hypotension has resulted from regional anesthesia. Local anesthetics produce vasodilation by blocking sympathetic nerves. The fetal heart rate also should be monitored continuously, and electronic fetal monitoring is highly advisable.
8.2 Lactation
Risk Summary
There are no data on the presence of chloroprocaine in human milk, the effects on the breastfed infant, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CLOROTEKAL® and any potential adverse effects on the breastfed infant from CLOROTEKAL® or from the underlying maternal condition.
8.5 Geriatric Use
Patients over 65 years, particularly those with hypertension, may be at increased risk of developing hypotension while undergoing spinal anesthesia with CLOROTEKAL®.
Clinical studies of CLOROTEKAL® did not include sufficient numbers of subjects 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general an elderly patient will have greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Warnings and Precautions (5) and Clinical Pharmacology (12.3)].
8.6 Hepatic and Renal Impairment
Since ester-type local anesthetics are hydrolyzed by plasma cholinesterase produced by the liver, the risk of toxic reactions might be greater in patients with advanced hepatic disease [see Clinical Pharmacology (12.3)].
This drug and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions might be greater in patients with impaired renal function [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use or to underventilation secondary to upward extension of spinal anesthesia. Hypotension is commonly encountered during the conduct of spinal anesthesia due to relaxation of sympathetic tone, and sometimes, contributory mechanical obstruction of venous return [see Warning and Precautions (5.1) and Adverse Reactions (6)].
In the case of accidental intravenous administration, the toxic effect occurs within 1 minute. In mice, the intravenous LD50 of chloroprocaine HCl is 97 mg/kg and the subcutaneous LD50 of chloroprocaine HCl is 950 mg/kg.
Management of Local Anesthetic Emergencies: the first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic injection. At the first sign of change, administration of CLOROTEKAL® must be stopped and oxygen should be administered [see Warning and Precautions (5.1)].
The first step in the management of convulsions, as well as underventilation or apnea, consists of immediate attention to the maintenance of a patient airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate or a benzodiazepine may be administered intravenously; the clinician should be familiar, prior to the use of anesthetics, with appropriate anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor dictated by the clinical situation (such as ephedrine to enhance myocardial contractile force). If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. Recovery has been reported after prolonged resuscitative efforts. Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated, after initial administration of oxygen by mask, if difficulty is encountered in the maintenance of a patient's airway or if prolonged ventilatory support (assisted or controlled) is indicated.
11 DESCRIPTION
CLOROTEKAL® is a sterile non pyrogenic local anesthetic.
The active ingredient in CLOROTEKAL® is chloroprocaine hydrochloride (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester, monohydrochloride), an ester local anesthetic, which is represented by the following structural formula:
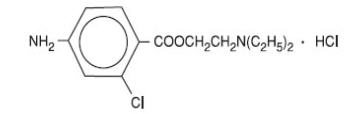
1 mL of solution for injection contains 10 mg of chloroprocaine hydrochloride, equivalent to 44.05 mg/5 mL (8.81 mg/mL) chloroprocaine. It also contains the following inactive ingredients: hydrochloric acid 1N (for pH adjustment), sodium chloride, water for injection. The pH of the solution is between 3.0 and 4.0. The osmolality of the solution is 270 – 300 mOsm/kg.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Chloroprocaine, like other local anesthetics, blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
12.2 Pharmacodynamics
Systemic absorption of local anesthetics produces effects on the cardiovascular and central nervous systems. At blood concentrations achieved with normal therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block and ultimately to cardiac arrest. In addition, with toxic blood concentrations myocardial contractility may be depressed and peripheral vasodilation may occur, leading to decreased cardiac output and arterial blood pressure.
Following systemic absorption, toxic blood concentrations of local anesthetics can produce central nervous system stimulation, depression, or both. Apparent central stimulation may be manifested as restlessness, tremors and shivering, which may progress to convulsions. Depression and coma may occur, possibly progressing ultimately to respiratory arrest.
However, the local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior stage of central nervous system stimulation.
12.3 Pharmacokinetics
The rate of systemic absorption of local anesthetic drugs is dependent upon the total dose concentration of drug administered, the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic injection.
The onset of action with chloroprocaine is rapid (usually 6 to 12 minutes), and the duration of anesthesia, depending upon the amount used and the route of administration.
Local anesthetics appear to cross the placenta by passive diffusion. However, the rate and degree of diffusion varies considerably among the different drugs as governed by: (1) the degree of plasma protein binding, (2) the degree of ionization, and (3) the degree of lipid solubility.
Fetal/maternal ratios of local anesthetics appear to be inversely related to the degree of plasma protein binding, since only the free, unbound drug is available for placental transfer. Thus, drugs with the highest protein binding capacity may have the lowest fetal/maternal ratios. The extent of placental transfer is also determined by the degree of ionization and lipid solubility of the drug. Lipid soluble, nonionized drugs readily enter the fetal blood from the maternal circulation.
Distribution
Depending upon the route of administration, local anesthetics are distributed to some extent to all body tissues, with high concentrations found in highly perfused organs such as the liver, lungs, heart, and brain. Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of hepatic or renal disease, addition of epinephrine, factors affecting urinary pH, renal blood flow, the route of administration, and the age of the patient.
Chloroprocaine plasma half-life in vitro is about 25 seconds, whereas the apparent half-life in vivo was found to be 3.1±1.6 min (range 1.5 to 6.4 min) in maternal plasma after intrapartum epidural anesthesia.
Metabolism
Chloroprocaine is rapidly metabolized in plasma by hydrolysis of the ester linkage by pseudocholinesterase. The hydrolysis of chloroprocaine results in the production of ß-diethylaminoethanol and 2-chloro-4-aminobenzoic acid, which inhibits the action of the sulfonamides [see Drug Interactions (7)].
Excretion
The kidney is the main excretory organ for most local anesthetics and their metabolites. Urinary excretion is affected by urinary perfusion and factors affecting urinary pH.
Pharmacokinetic after intrathecal administration
Plasma concentrations of chloroprocaine and 2-chloro-4-aminobenzoic acid (ACBA) after intrathecal administration of 50 mg dose of CLOROTEKAL® are reported in Table 3.
| Analyte | chloroprocaine | ACBA |
|---|---|---|
| Dose group | 50 mg | 50 mg |
| N=15 | N=15 | |
| Mean ± SD is shown; BLQL: below the quantification limit (4.0 ng/mL) | ||
| Pre-dose (0) | BLQL | BLQL |
| 5 min post-dose | BLQL | 24.9±20.3 |
| 10 min post-dose | BLQL | 75.8±67.6 |
| 30 min post-dose | BLQL | 97.6±61.7 |
| 60 min post-dose | BLQL | 78.4±48.4 |
Specific Populations
Renal Impairment
Chloroprocaine is known to be substantially excreted by the kidney [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate carcinogenic potential of chloroprocaine have not been conducted.
14 CLINICAL STUDIES
14.1 Study 1
A Phase 2 single-center, prospective, randomized, observer-blind study evaluated the efficacy and the tolerability of chloroprocaine 30, 40, and 50 mg after spinal injection in 45 patients (27 males and 18 females) undergoing short duration (< 40 minutes) lower limb surgery. The mean age was 41 years (range 19 to 63).
Efficacy was determined by proportion of patients who were able to complete the surgical procedure without the need for supplementary intravenous analgesic or sedation drugs.
Efficacy results
Neither rescue anesthesia nor rescue analgesia was required for subjects randomized to chloroprocaine 50 mg. Three subjects in the 30 mg dose group and three subjects in the 40 mg dose group required intraoperative rescue medications. Duration data such as time from injection to surgery end, etc., for the chloroprocaine 50 mg-administered subjects follow:
| Mean | Median | Minimum | Maximum | |
|---|---|---|---|---|
| Time from injection to surgery start | 22 | 20 | 11 | 42 |
| Time from injection to surgery end | 41 | 40 | 24 | 60 |
| Time from injection to resolution of motor block | 100 | 104 | 56 | 146 |
| Time from surgery start to surgery finish | 20 | 20 | 5 | 40 |
The maximum surgical duration in the 50 mg dose group was 40 minutes and 93% (14 of 15) of the 50 mg dose group had surgical procedures ≤ 30 minutes.
14.2 Study 2
A Phase 3, multicenter, prospective, randomized, observer-blind study evaluated the safety and the efficacy of 50 mg of chloroprocaine 10 mg/mL in intrathecal anesthesia versus 10 mg of bupivacaine 0.5%, in 130 patients (69 males and 61 females) undergoing short duration (< 40 minutes) low abdominal surgery (gynecological or urological) and lower limb surgery that required T10 metameric level of sensory block and identical anesthesia procedures.
The mean age was 45 years (range 18 to 78) in the chloroprocaine group and 51 years (range 20 to 79) in the bupivacaine group. Each patient received a single dose of anesthetic (50 mg of chloroprocaine or 10 mg of bupivacaine) according to the randomization plan. Sixty-six subjects were randomized to the chloroprocaine group. Sixty-four subjects were randomized to the bupivacaine group.
Efficacy was determined by proportion of patients who were able to complete the surgical procedure without the need for rescue intravenous analgesic or sedation drugs.
Efficacy results
Six of 66 subjects (9%) in the chloroprocaine group required rescue compared to 6 of 64 (9%) in the bupivacaine group. Duration data such as time from injection to surgery end, etc., for the chloroprocaine-administered subjects follow:
| Mean | Median | Minimum | Maximum | |
|---|---|---|---|---|
| Time from injection to surgery start | 16 | 15 | 3 | 42 |
| Time from injection to surgery end | 39 | 34 | 9 | 90 |
| Time from injection to resolution of motor block | 101 | 100 | 40 | 194 |
| Time from surgery start to surgery finish | 23 | 20 | 3 | 78 |
Only two subjects had surgical procedures lasting over 60 minutes, and both required intraoperative rescue medications.
16 HOW SUPPLIED/STORAGE AND HANDLING
The CLOROTEKAL® is supplied as a 50mg/5mL (10 mg/mL) equivalent to 44.05 mg/5 mL (8.81 mg/mL) chloroprocaine Type I glass ampules, stored in cartons containing 10 single-dose ampules.
| NDC | REF | Size |
| 0264-7055-10 | D7055 | 5mL |
The product is intended for intrathecal administration.
Solutions which are discolored or which contain particulate matter should not be administered.
17 PATIENT COUNSELING INFORMATION
Inform patients in advance that chloroprocaine-containing products can cause temporary loss of sensation or motor activity, usually in the lower half of the body, following proper administration of spinal anesthesia.
Rx only
Clorotekal® is a registered trademark of Sintetica S.A.
Manufactured by:
Sintetica S.A.
Switzerland
Manufactured for:
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Made in Switzerland
LD-578-1
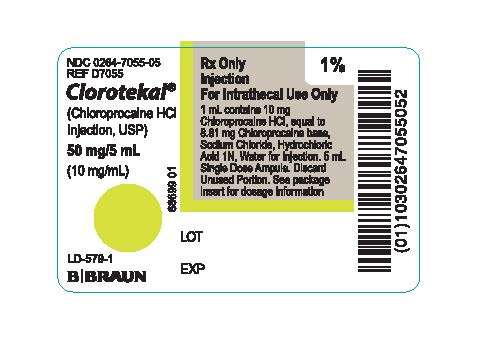
PRINCIPAL DISPLAY PANEL - 5 mL Ampule Label
NDC 0264-7055-05
REF D7055
Clorotekal®
(Chloroprocaine HCl
Injection, USP)
50 mg/5 mL
(10 mg/mL)
LD-579-1
Rx Only
Injection
For Intrathecal Use Only
1 mL contains 10 mg Chloroprocaine HCI, equal to 8.81 mg Chloroprocaine base, Sodium Chloride, Hydrochloric Acid 1N, Water for Injection. 5 mL Single Dose Ampule. Discard Unused Portion. See package insert for dosage information
68699 01
LOT
EXP
1%
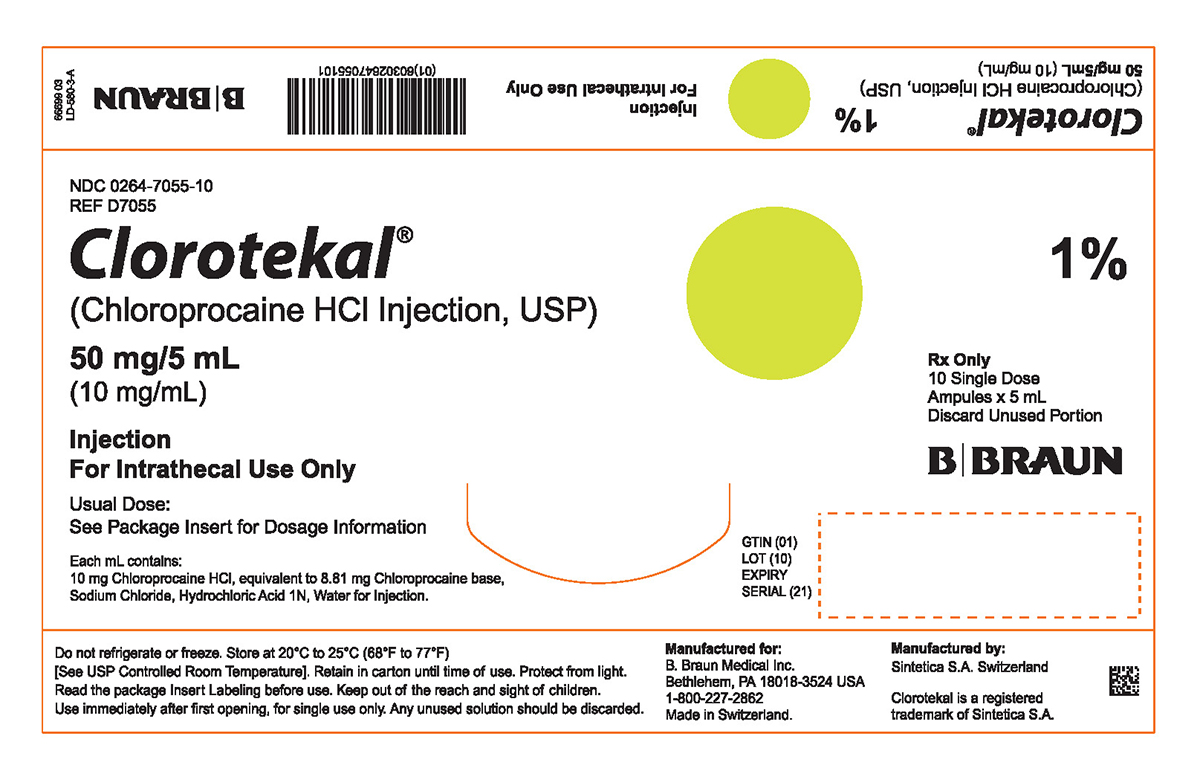
PRINCIPAL DISPLAY PANEL - 5 mL Ampule Carton (A)
NDC 0264-7055-10
REF D7055
Clorotekal®
(Chloroprocaine HCl Injection, USP)
50 mg/5 mL
(10 mg/mL)
Injection
For Intrathecal Use Only
Usual Dose:
See Package Insert for Dosage Information
Each mL contains:
10 mg Chloroprocaine HCl, equivalent to 8.81 mg Chloroprocaine base,
Sodium Chloride, Hydrochloric Acid 1N, Water for Injection.
1%
Rx Only
10 Single Dose
Ampules x 5 mL
Discard Unused Portion
GTIN (01)
LOT (10)
EXPIRY
SERIAL (21)
Do not refrigerate or freeze. Store at 20°C to 25°C (68°F to 77°F)
[See USP Controlled Room Temperature]. Retain in carton until time of use. Protect from light. Read the package Insert Labeling before use. Keep out of the reach and sight of children. Use immediately after first opening, for single use only. Any unused solution should be discarded.
Manufactured for:
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Made in Switzerland.
Manufactured by:
Sintetica S.A. Switzerland
Clorotekal is a registered trademark of Sintetica S.A.
66699 03
LD-580-3-A
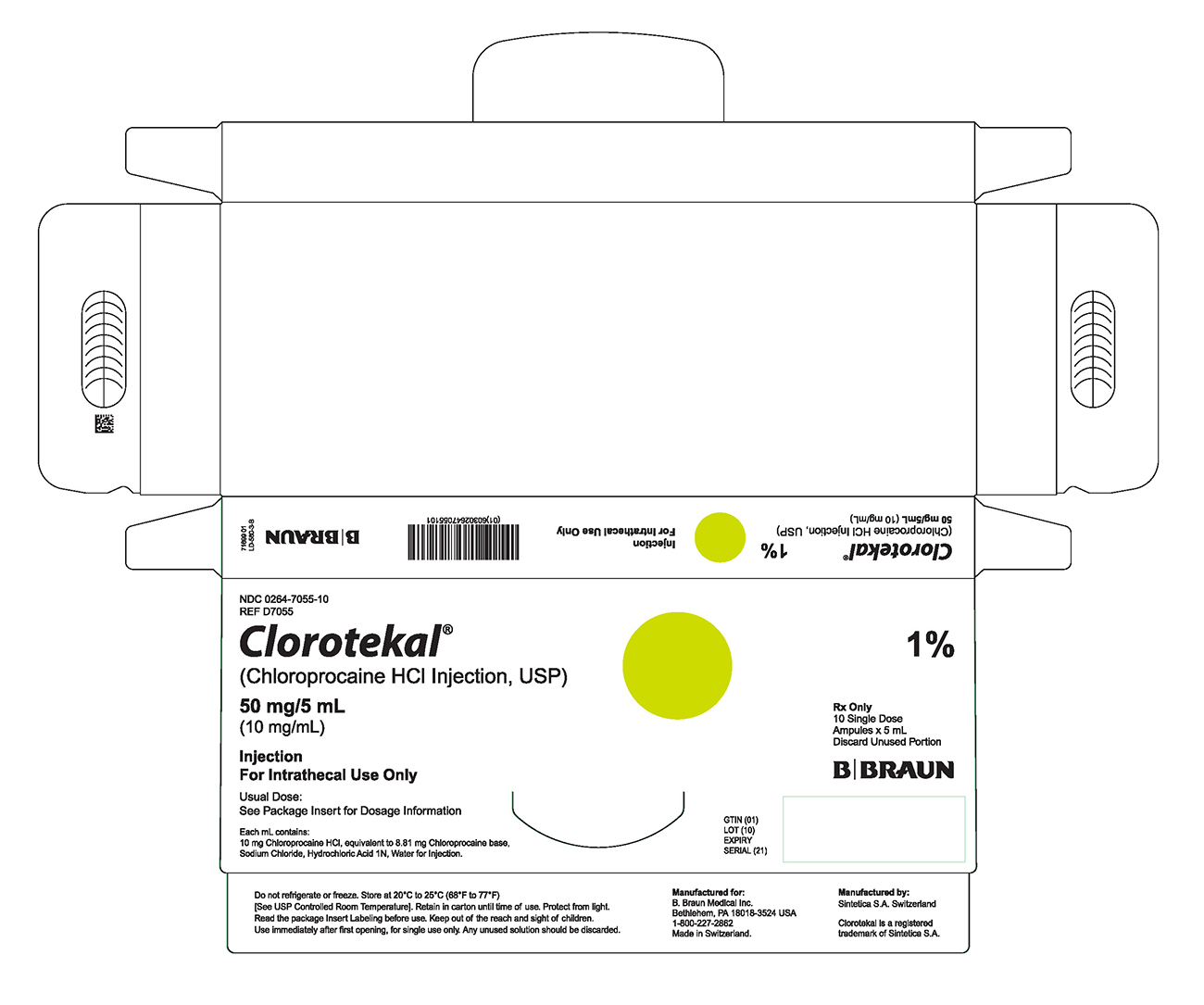
LD-580-3-A
PRINCIPAL DISPLAY PANEL - 5 mL Ampule Carton (B)
NDC 0264-7055-10
REF D7055
Clorotekal®
(Chloroprocaine HCl Injection, USP)
50 mg/5 mL
(10 mg/mL)
Injection
For Intrathecal Use Only
Usual Dose:
See Package Insert for Dosage Information
Each mL contains:
10 mg Chloroprocaine HCl, equivalent to 8.81 mg Chloroprocaine base,
Sodium Chloride, Hydrochloric Acid 1N, Water for Injection.
1%
Rx Only
10 Single Dose
Ampules x 5 mL
Discard Unused Portion
GTIN (01)
LOT (10)
EXPIRY
SERIAL (21)
Do not refrigerate or freeze. Store at 20°C to 25°C (68°F to 77°F)
[See USP Controlled Room Temperature]. Retain in carton until time of use. Protect from light. Read the package Insert Labeling before use. Keep out of the reach and sight of children. Use immediately after first opening, for single use only. Any unused solution should be discarded.
Manufactured for:
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Made in Switzerland.
Manufactured by:
Sintetica S.A. Switzerland
Clorotekal is a registered trademark of Sintetica S.A.
66699 03
LD-580-3-B
LD-580-3-B