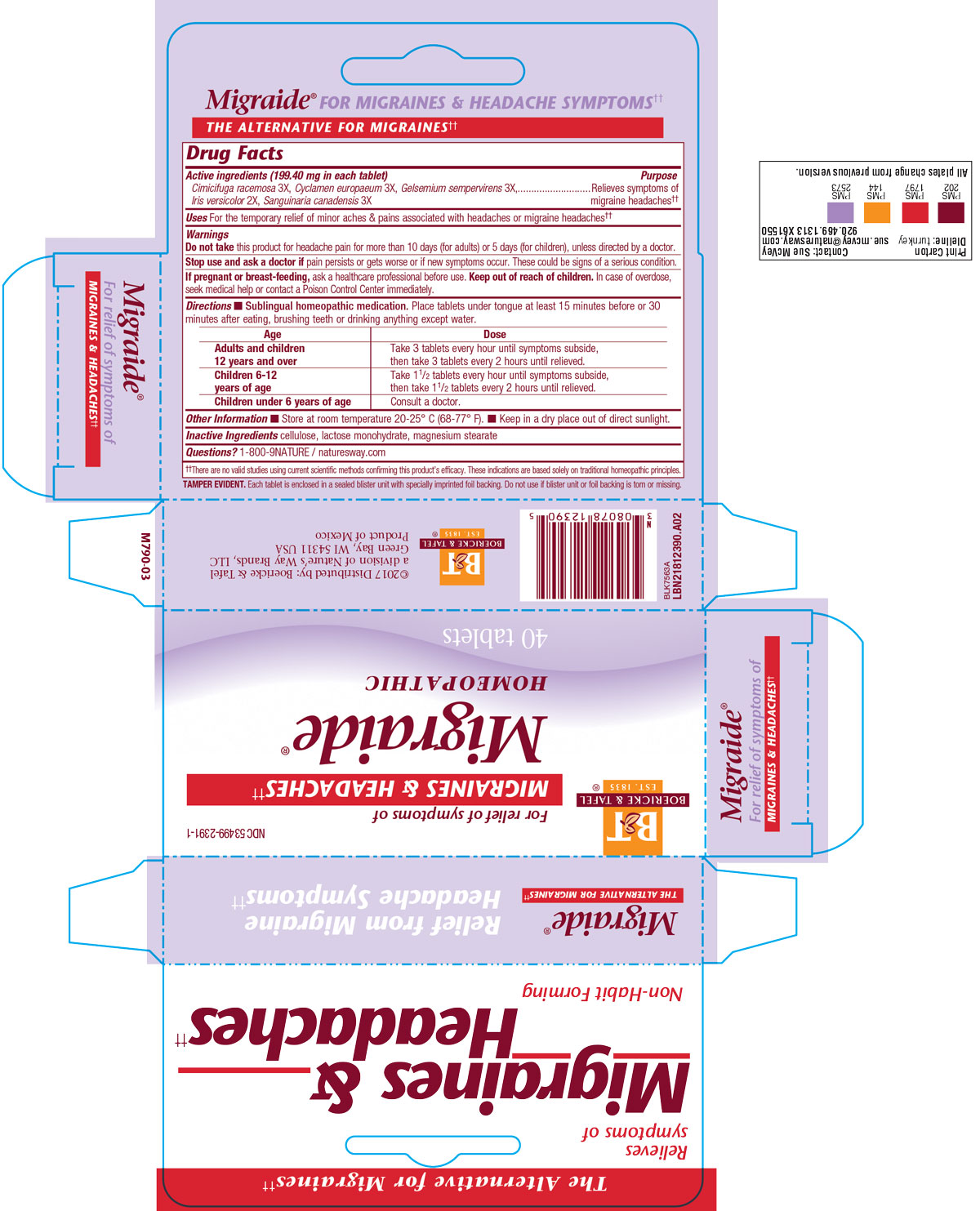
Active Ingredient
Cimicifuga racemosa 3X
Cyclamen europaeum 3X
Gelsemium sempervirens 3X
Iris versicolor 2X
Sanguinaria canadensis 3X
Dosage & Administration
Directions: Sublingual homeopathic medication.
Place tablets under tongue at least 15 minutes before or half hour after eating, brushing teeth, or drinking anything except water.
Adults and children 12 years of age and older: Take 3 tablets every hour until symptoms subside, then take 3 tablets every 2 hours until relieved.
Children 6 to 12: Take 1 1/2 tablets every hour until symptoms subside, then take 1 1/2 tablets every 2 hours until relieved.
Children under 2: Consult a doctor.
Purpose
For the temporary relief of minor aches and pains associated with headaches or migraine headaches.
Indications & Usage
For the temporary relief of minor aches and pains associated with headaches or migraine headaches.
Warnings
Do not take this product for headache pain for more than 10 days (for adults) or 5 days (for children) unless directed by a doctor.