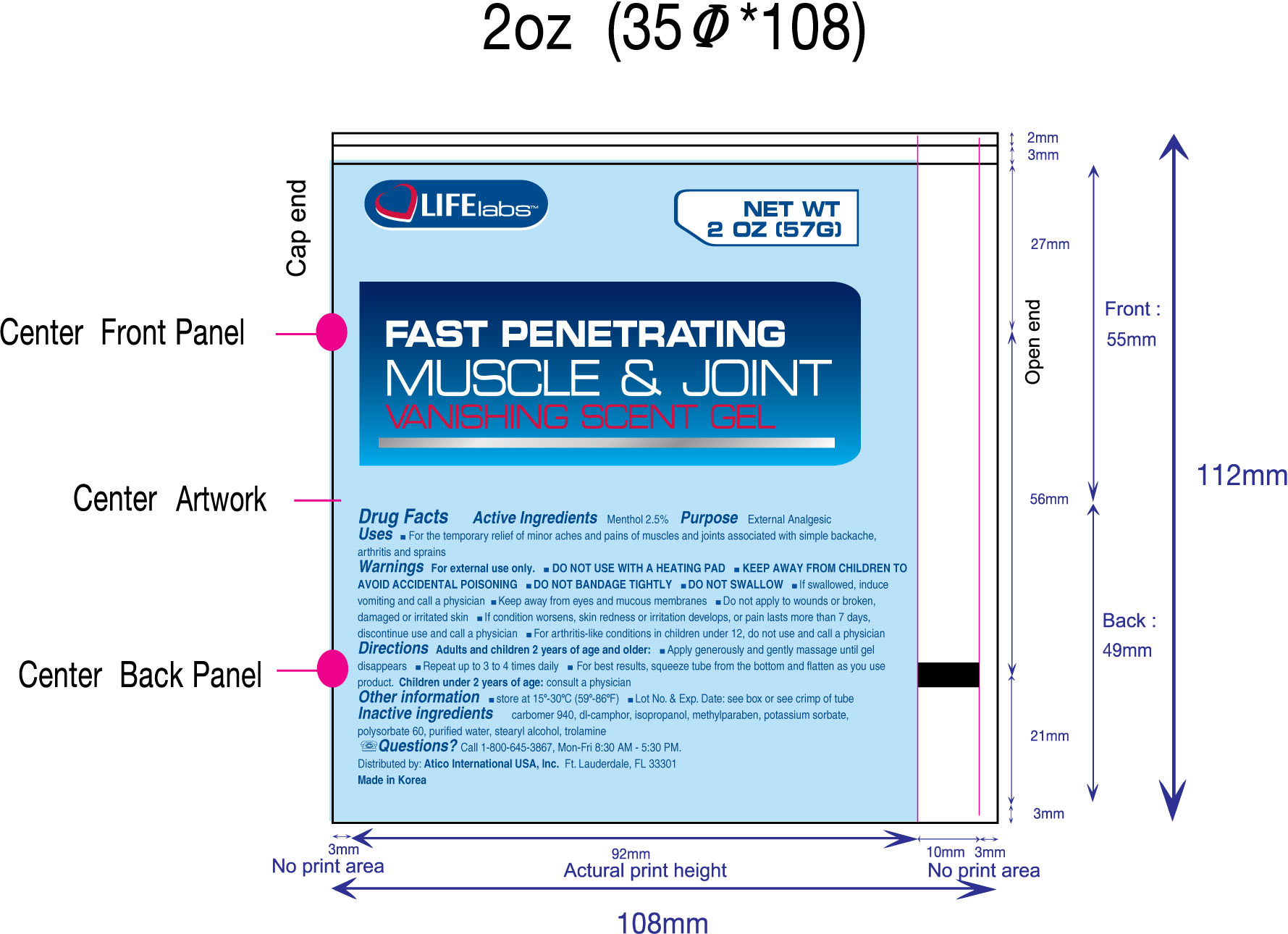
Warnings
For external use only
- DO NOT USE WITH A HEATING PAD
- KEEP AWAY FROM CHILDREN TO AVOID ACCIDENTAL POISONING
- DO NOT BANDAGE TIGHTLY
- DO NOT SWALLOW
- If swallowed, induce vomiting and call a physician.
- Keep away from eyes and mucous membranes
- Do not apply to wounds or broken, damaged or irritated skin.
- If condition worsens, skin redness or irritation develops, or pain lasts more than 7 days, discontinue use and call a physician
- For arthritis-like conditions in children under 12, do not use and call a physician.
Directions
Adults and children 2 years of age and older
- Apply generously and gently massage until gel disappears
- Repeat up to 3 to 4 times daily
- For best results, squeeze tube from the bottom and flatten as you use product.
Other information
Store between 15oC - 30oC (59oF-86oF)
Lot No. and Exp. Date: see box or see crimp of tube
Inactive Ingredients
carbomer 940, dl-camphor, isopropanol, methylparaben, potassium sorbate, polysorbate 60, purified water, stearyl alcohol, trolamine