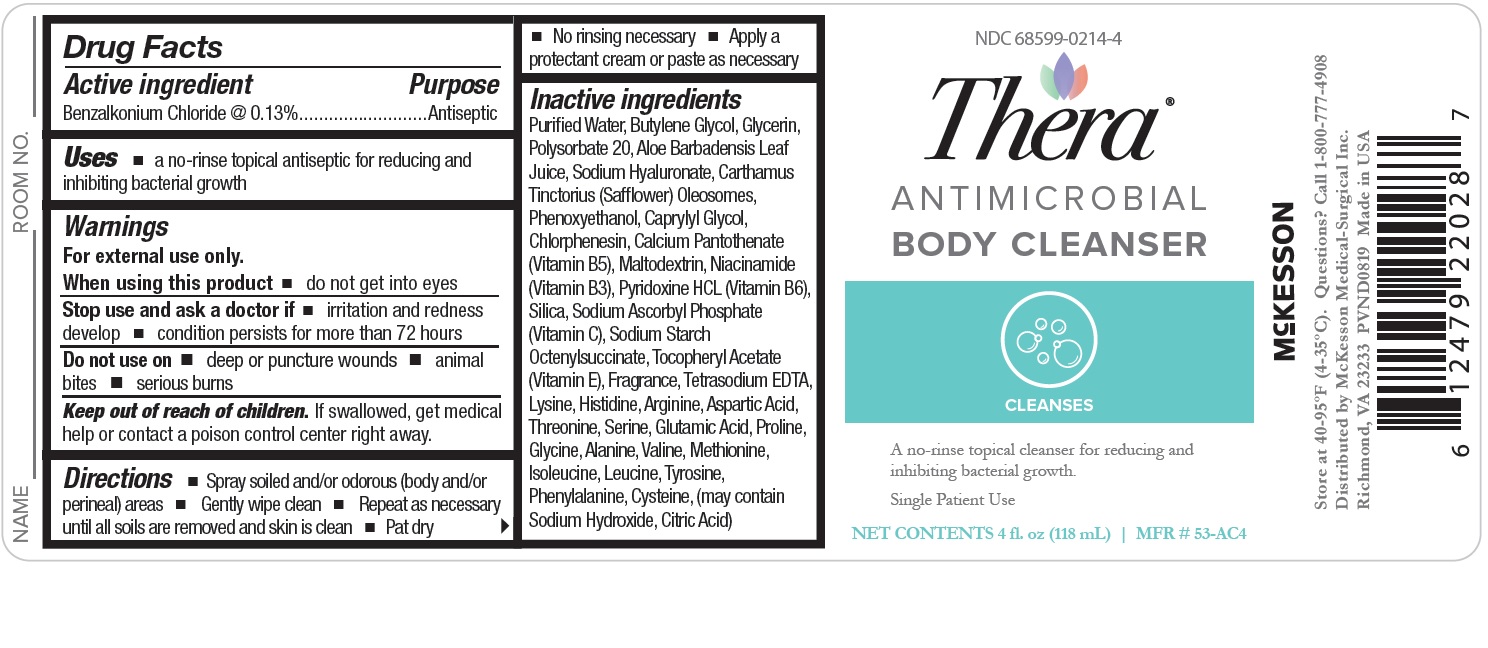
Warnings
For external use only.
When using this product
- do not get into eyes
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- irritation and redness develop
- condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away.
Directions
- Spray soiled and/or odorous (body and/or perineal) areas
- Gently wipe clean
- Repeat as necessary until all soils are removed and skin is clean
- Pat dry
- No rinsing necessary
- Apply a protectant cream or paste as necessary
Inactive Ingredients:
Purified Water, Butylene Glycol, Glycerin, Polysorbate 20, Aloe Barbadensis Leaf Juice,
Sodium Hyaluronate, Carthamus Tinctorius (Safflower) Oleosomes, Phenoxyethanol, Caprylyl Glycol,
Chlorphenesin, Calcium Pantothenate (Vitamin B5), Maltodextrin, Niacinamide (Vitamin B3),
Pyridoxine HCL (Vitamin B6), Silica, Sodium Ascorbyl Phosphate (Vitamin C),
Sodium Starch Octenylsuccinate, Tocopheryl Acetate (Vitamin E), Fragrance, Tetrasodium EDTA,
Lysine, Histidine, Arginine, Aspartic Acid, Threonine, Serine, Glutamic Acid, Proline,
Glycine, Alanine, Valine, Methionine, Isoleucine, Leucine, Tyrosine, Phenylalanine, Cysteine,
(may contain Sodium Hydroxide, Citric Acid)