Eyesaline
Active ingredient
Sterile Water 99%
Eyesaline
Purpose
Eyewash
Eyesaline
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Stop use and ask a doctor if
- you experience eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Eyesaline
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyesaline
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
Eyesaline
Questions
1-800-430-5490 Honeywell Sadety Products USA, Inc. Smithfield, RI 02917
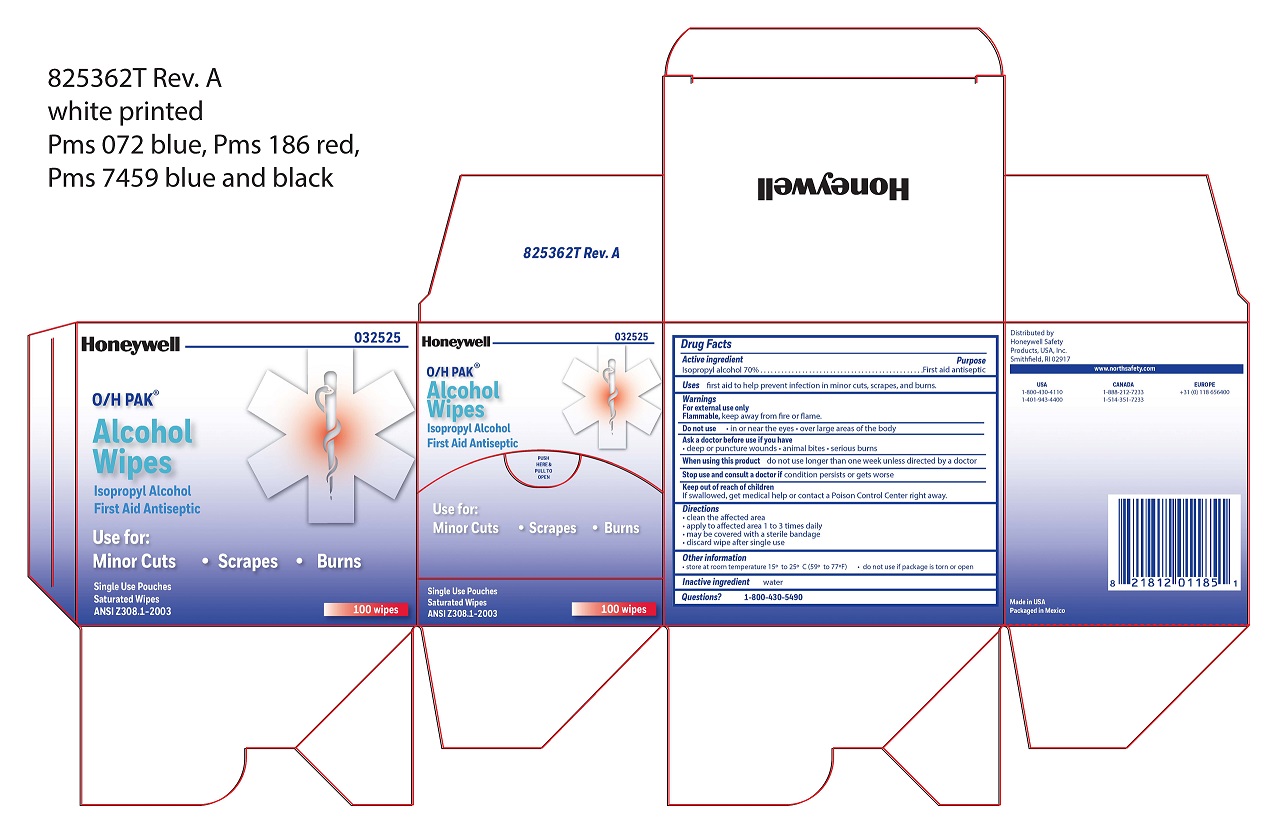
Alcohol Wipe
Active ingredient
Isopropyl alcohol 70%
Alcohol Wipe
Purpose
First aid antiseptic
Alcohol Wipe
Uses
- first aid to help prevent infection in minor cuts, scrapes, and burns
Alcohol Wipe
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burn
When using this product
- do not use longer than one week unless directed by a doctor
Stop use and consult a doctor
- if condition persists or gets worse
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Alcohol Wipe
Directions
- clean the affected area
- apply wipe to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard wipe after single use
Alcohol Wipe
Other information
store at room temperature 15
0 to 25
0 C (59
0 to 77
0F)
Alcohol Wipe
Inactive ingredient
water
Alcohol Wipe
Questions
1-800-430-5490
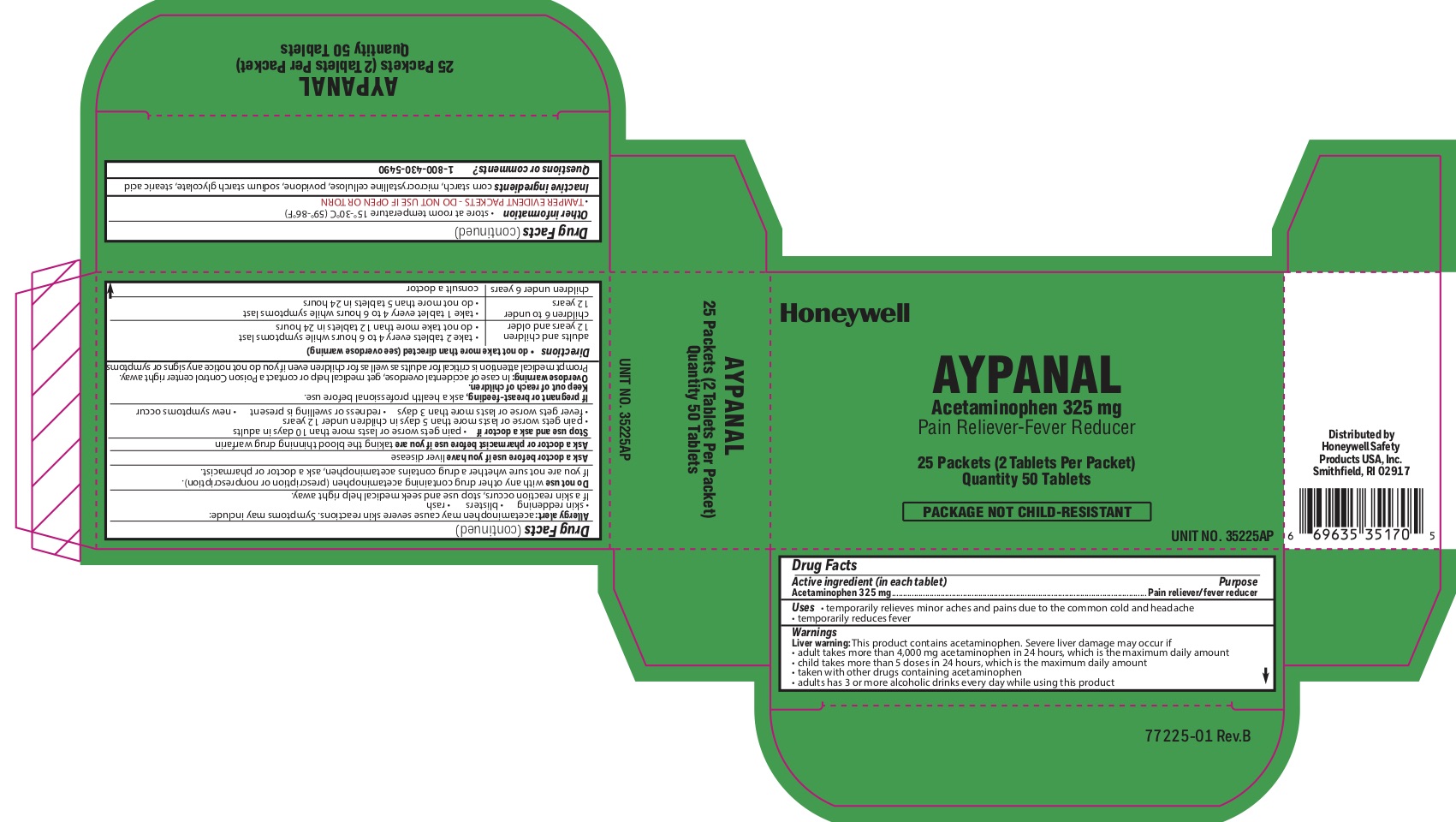
Aypanal
Active ingredient
Acetaminophen 325 mg
Aypanal
Purpose
Pain reliever/ fever reducer
Aypanaly
Uses
- temporarily relieves minor aches and pains due to the common cold and headache - temporarily reduces fever
Aypanal
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product:
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
- If a skin rash occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
Ask a doctor or pharmacist before use if
- you are taking the blood thinning drug warfarin
Stop using and ask a doctor if
- pain gets worse or lasts more than 10 days in adults
- pain gets worse or lasts more than 5 days in children under 12 years
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
If pregnant or breast-feeding
If pregnant or breast-feeding, ask a health professional before use.
Overdose warning
- In case of accidental overdose, get medical help or contact a Poison Control Center right away.
- Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Keep out of reach of children.
Keep out of reach of children.
Aypanal
Directions
do not take more than directed (see overdose warning)
adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
children under 6 years consult a doctor
Aypanal
Other information
- store at room temperature 15
0 to 30
0 C (59
0 - 86
0 F)
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
Aypanal
Inactive ingredients
corn starch, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
Aypanal
Questions
1-800-430-5490
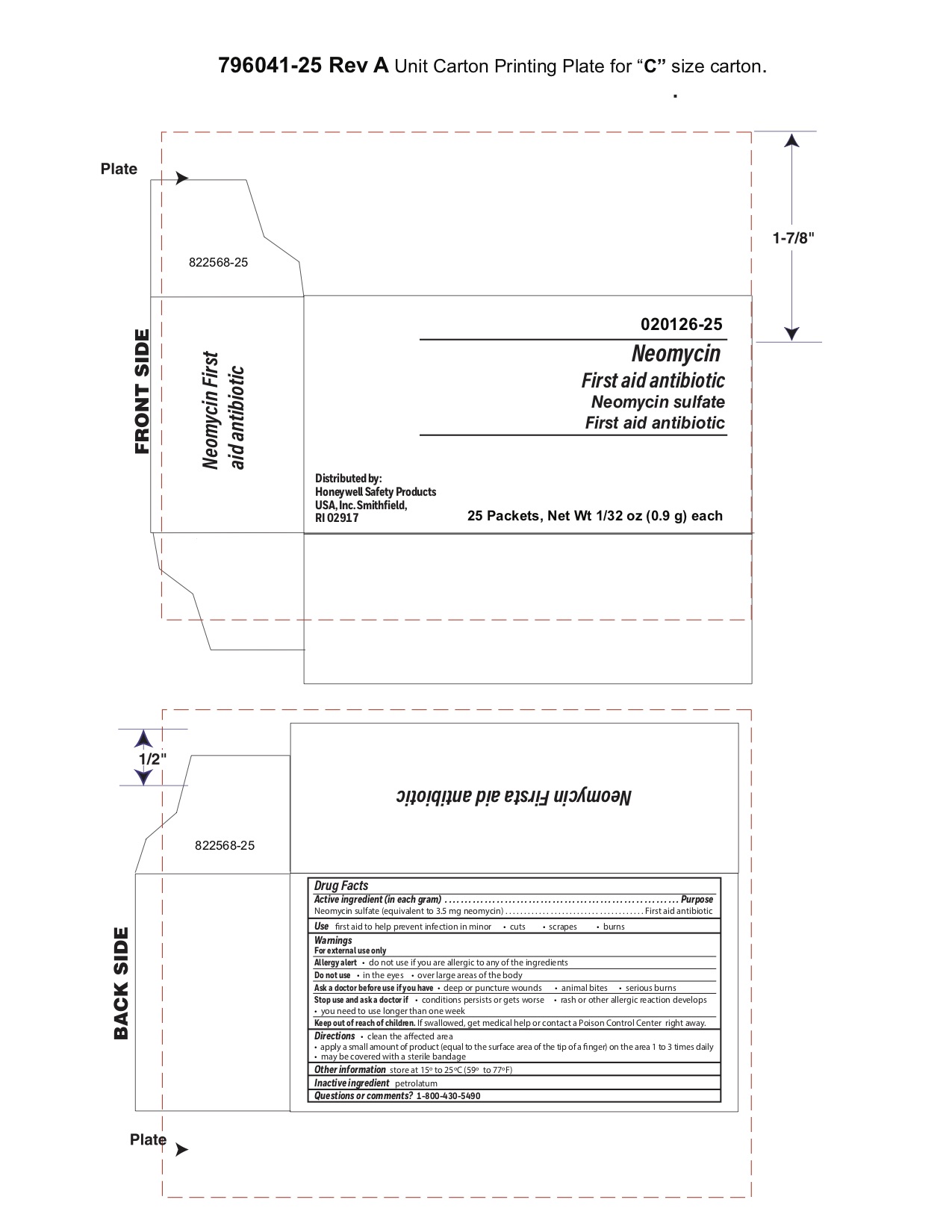
Neomycin
Active ingredient
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Neomycin
Purpose
First aid antibiotic
Neomycin
Uses
- first aid to help prevent infection in - minor cuts - scrapes - burns
Neomycin
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Stop use and ask a doctor if
- the condition persists or gets worse
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
Do not use
- in the eyes
- over large areas of the body
Neomycin
Direction
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Neomycin
Other information
store at 15
0 to 25
0 C (59
0 to 77
0 F)
Neomycin
Inactive ingredient
petrolatum
Neomycin
Questions?
1-800-430-5490
Antiseptic Spray
Active ingredient
Benzalkonium chloride 0.13%
Antiseptic Spray
Purpose
First aid antiseptic
Antiseptic Spray
Uses
- first aid to help prevent infection in minor cuts, scrapes and burns
Antiseptic Spray
Warnings
For external use only
Do not use
- in or near the eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not use longer than one week unless directed by a doctor
Stop use and ask a doctor if
- the condition persists or gets worse
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
Antiseptic Spray
Directions
- clean the affected area
- spray a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
Antiseptic Spray
Other information
- shake well
- store at room temperature 15
0-30
0 C (59
0 -86
0 F)
Antiseptic Spray
Inactive ingredients
diazolidinyl urea, edetate disodium, glycerin, hypromellose, methylparaben, octoxynol 9, propylene glycol, propylparaben, trolamine, water
Antiseptic Spray
Questions
1-800-430-5490
Burn Spray
Active ingredient
Benzethonium chloride 0.2% w/w
Benzocaine 10% w/w
Menthol 0.33% w/w
Burn Spray
Purpose
Topical antiseptic
Topical anesthetic
Topical antiseptic
Burn Spray
Uses
for the temporary relief of pain and itching and helps protect against infection in:
- minor cuts and scrapes
- burns
- sunburn
- insect bites
- minor skin irritations
Burn Spray
Warnings
For external use only
Flammable
- keep away from fire or flame
- contents under pressure
- do not puncture or incinerate container
- do not expose to temperatures above 120 0 F
Do not use
- in or near the eyes or other mucous membranes
- in case of serious burns
- in case of deep or puncture wounds
- for prolonged period of time
- on large portion of the body
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- condition clears up and recurs within a few days
- redness, swelling, or irritation occurs
Keep out of the reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Burn Spray
Directions
- clean the affected area
- shake can well before using
- hold 4 - 6 inches from surface and spray area until wet
- may be covered with a sterile bandage, if bandaged let dry first
- for adult institutional use only
- not intended for use on children
Burn Spray
Other information
- avoid inhaling
- use only as directed
- intentional misuse by deliberately concentrating or inhaling the contents may be harmful or fatal
Burn Spray
Inactive ingredients
dipropylene glycol, isobutane, n-butane, propane
Hand Sanitizer
Active ingredient
Ethyl alcohol 62%
Hand Santizer
Purpose
Antiseptic handwash
Hand Sanitizer
Uses
- for hand washing to decrease bacteria on skin
- recommended for repeated use
Hand Sanitizer
Warnings
For external use only
Flammable, keep away from fire or flame
When using this product
- do not use in the eyes
- discontinue use if irritation and redness develops. If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Hand Sanitizer
Directions
- wet hands thoroughly with product and allow to dry without wiping
Hand Sanitizer
Other information
store at 15
0 to 25
0 C (59
0 to 77
0 F)
Hand Sanitizer
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, dl-alpha tocopheryl acetate, fragrance, PEG-60 almond glycerides, propylene glycol, purified water, triisopropanolamine
Hand Sanitizer
Questions or Comments?
1-800-275-3433 info@waterjel.com www.waterjel.com
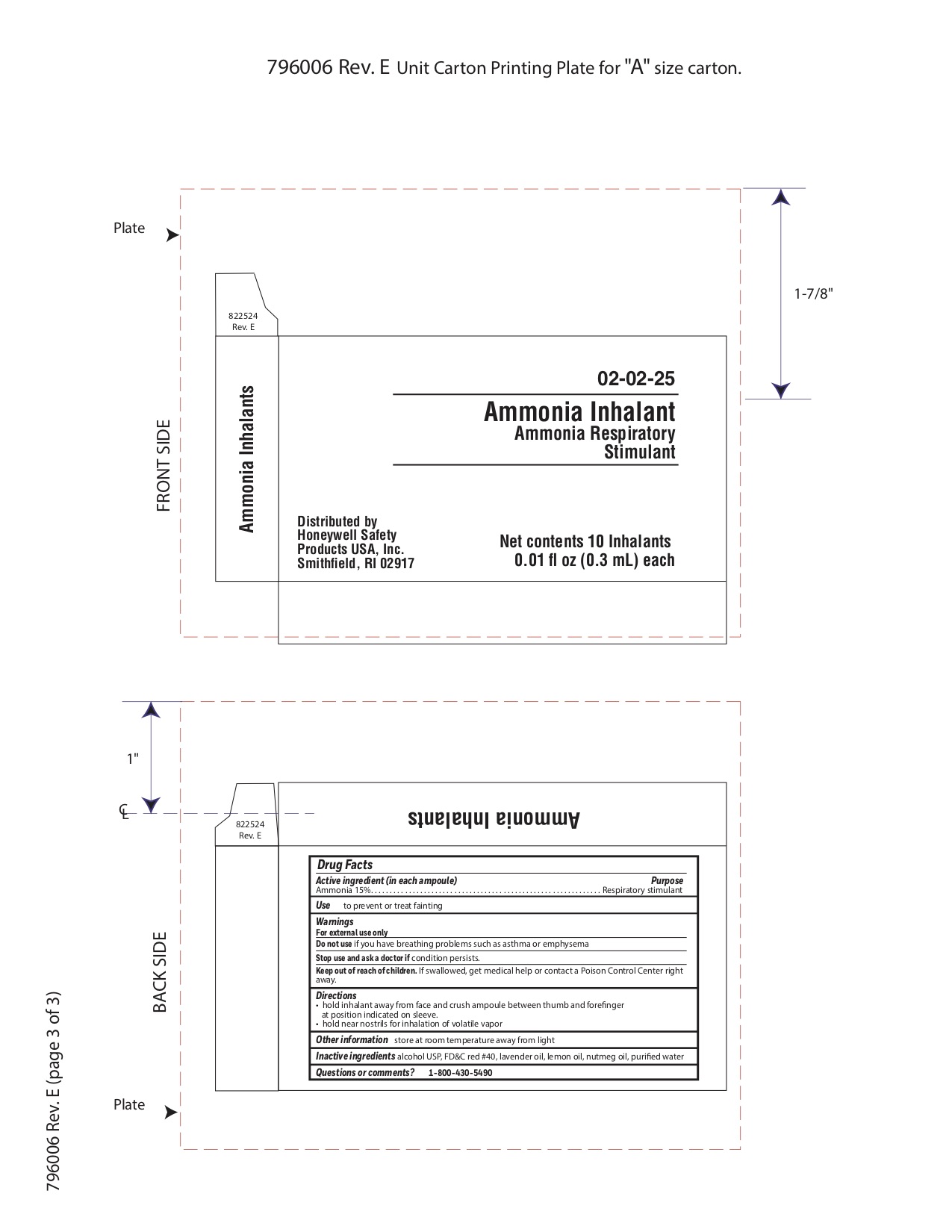
Ammonia
Active ingredient
Ammonia 15%
Ammonia
Purpose
Respiratory stimulant
Ammonia
Uses
- to prevent or treat fainting
Ammonia
Warnings
For external use only
Do not use
- if you have breathing problems such as asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Ammonia
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia
Other information
- store at room temperature away from light
Ammonia
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Ammonia
Questions or Comments?
1-800-430-5490
Cedaprin
Active ingredient
Ibuprofen 200 mg (NSAID)
*(nonsteroidal anti-inflammatory drug)
Cedaprin
Purposse
Pain reliever/fever reducer
Cedaprin
Uses
temporarily relieves minor aches and pains due to:
- headache
- muscular aches
- minor pain of arthritis
- toothache
- backache
- the common cold
- menstrual cramps
- temporarily reduces fever
Cedaprin
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning:
- NSAID's, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to ibuprofen or any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- you have problems or serious side effectsfrom taking pain relievers or fever reducers
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma or had a stroke
- you are taking a diuretic
Ask a doctor or a pharmacist before use if you are
- taking aspirin for heart attack or stroke, because ibuprofen may decrease the benefit of aspirin
- under a doctors care for any serious condition
- taking any other drug
When using this product,
take with food or milk if stomach upset occurs
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in oe part or side of body
- slurred speech
- leg swelling
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appea
If pregnant or breast-feeding,
ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away(1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Cedaprin
Directions
-
do not take more than directed
-
the smallest effective dose should be used
- adult and children 12 years of age and over:
- take 1 tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
- children under 12 years: ask a doctor
Cedaprin
Other information
store between 15
0 -30
0 C (59
0 -86
0 F)
avoid excessive heat and humidity
TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
cedaprin
Inactive ingredients
hypromellose, lactose monohydrate, opadry II 31K, povidone K-30, ferric oxide red, silicon dioxide, starch, stearic acid, titanium dioxide, triacetin
Cedaprin
Questions or Comments?
1-800-430-5490
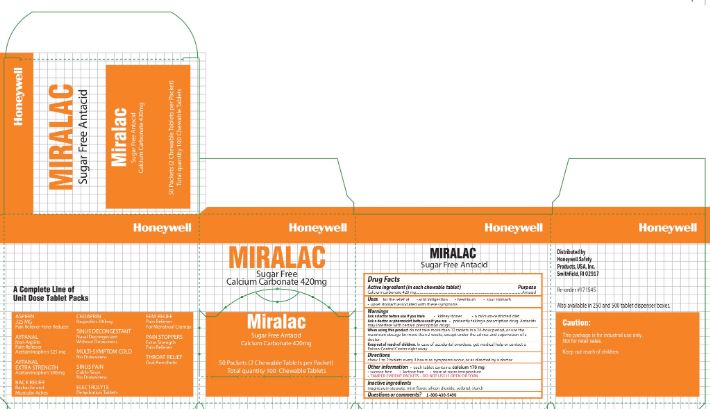
Miralac
Active ingredient
Calcium carbonate 420 mg
Miralac
Uses
for the relief of
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
Miralac
Warnings
Ask a doctor before use if you have
- kidney stones
- calcium-restricted diet
Ask a doctor before use if you are
presently taking a prescription drug. Antacids may interfere with certain prescription drugs
When using this product
do not take more than 12 tablets in a 24- hour period, or use the maximum dosage
Keep out of the reach of children.
In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Miralac
Directions
chew 1 to 2 tablets every 4 hours as symptoms occur, or as directed by a doctor.
Miralac
Other information
- each tablet contains: calcium 170 mg
- sucrose free
- lactose free
- store at room temperature
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
Miralac
Inactive ingredients
magnesium stearate, mint flavor, silicon dioxide, sorbitol, starch
Miralac
Questions or Comments
1-800-430-5490
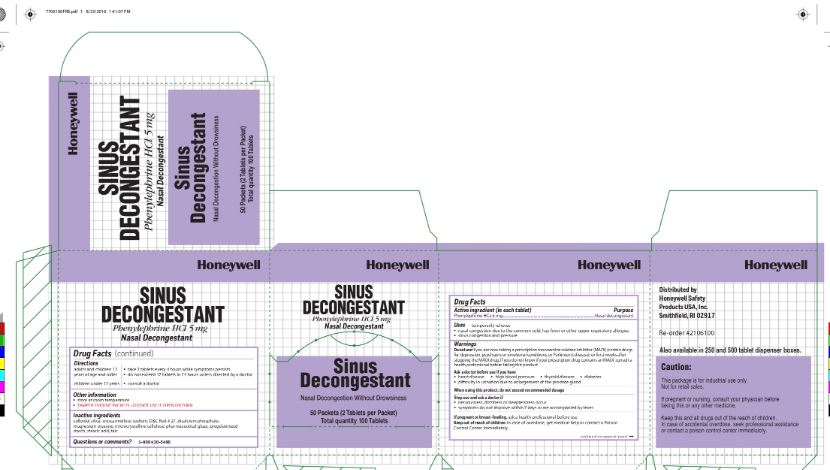
Sinus decongestant
Active ingredient
Phenylephrine HCl 5 mg
Sinus Decongestant
Purpose
Nasal decongestant
Sinus Decongestant
Uses
Temporarily relieves
- nasal congestion due to:the common cold, hay fever, or other respiratory allergies
- sinus congestion and pressure.
Sinus Decongestant
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, consult a health professional before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
When using this product
do not exceed recommended dosage
Stop use and ask a doctor if
- nervousness
- dizziness
- sleeplessness occur
- symptoms do not improve within 7 days or are accompanied by fever
If pregnant or breast feeding
ask a health professional
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center immediately
Sinus Decongestant
Directions
- adults and children 12 years of age and over:
- take 2 tablets every 4 hours, not to exceed 12 tablets in 24 hours
- children under 12 years, consult a doctor
Sinus Decongestant
Other information
- store at room temperature
- Tamper Evident Packets
- do not use if open or torn
Sinus Decongestant
Inactive ingredients
colloidal silica, croscarmellose sodium, D and C Red # 27 Lake, dicalcium phosphate, magnesium stearate, microcrystalline cellulose, pharmaceutical glaze, pregelatinized starch, stearic acid, talc
Sinus Decongestant
Questions or Comments
1-800-430-5490
4392
SF00004422 Kit Contents
1 1X3 PLASTIC 100/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 SWIFT KNUCKLE 40/BX
1 1 X 3 WOVEN 100/BOX
3 NEOMYCIN ANTIBIOTIC 10 PER
1 AMMONIA INHALANTS 10 PER
2 EYE DRESS PKT W/4 ADH STRIPS
1 TOURNIQUET, 1 PER
1 WIRE SPLINT 1 PER
1 ADH BAND, EXTRA LARGE, 6 PER
1 ALCOHOL PREP PADS 10P
1 O/H PUMP ANTISEPTIC 2 OZ ID F
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 FIRST AID GUIDE ASHI
2 TAPE ADHESIVE 1"X 5 YD PLSTC
10 HAND SANITIZER 0.9G WJ BULK
4 GAUZE CLEAN-WRAP BDGE N/S 2"
4 GAUZE CLEAN-WRAP BDGE N/S 4"
4 BLOODSTOPPER
1 NON-ADHERENT PADS 2"X3" 10'S
1 GZE PADS STERILE 2"X 2" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 COTTON TIPS 100 PER VIAL
1 AYPANAL NON-ASP IND 2/ENV 100
1 CEDAPRIN (IBUPROFEN) 2ENV 100
1 MIRALAC TABS IND PK 2/ENV 100
1 SINUS DECONGESTANT 2/ENV 100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 400 EMPTY KIT BLANK
1 POCKET INSERT RED #400 KIT 5R
1 TONGUE BLADES SR WRAPPED 6'S
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
2 x2 PR LRG NITRILE GLVES ZIP BAG
2 WATER-JEL BURN DRESSING 4 X 4
8 CORNER STYROFOAM 3X3X3
2TRI BNDG NON WOVEN 40"X40"X56"
2 COLD PACK UNIT 4"X6" BULK
1 RED BIO BAGS 2/BX
Eyesaline
Principal Display Panel

Alcohol Wipe
Principal Display Panel
Aypanal
Principal Display Panel
Neomycin
Principal Display Panel
Antiseptic Spray
Principal Display Panel

Burn Spray
Principal Display Panel

Hand Sanitizer
Principal Display Panel

Ammonia
Principal Display Panel
Cedaprin
Principal Display Panel

Miralac
Principal Display Panel
Sinus Decongestant
Principal Display Panel
4390 Kit Label
FAK4SHLF-CLSB

4390 Kit Contnets
FAK4SHLF-CLSB
1 1X3 PLASTIC 100/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 SWIFT KNUCKLE 40/BX
1 1 X 3 WOVEN 100/BOX
3 NEOMYCIN ANTIBIOTIC 10 PER
1 AMMONIA INHALANTS 10 PER
2 EYE DRESS PKT W/4 ADH STRIPS
1 TOURNIQUET, 1 PER
1 WIRE SPLINT 1 PER
1 ADH BAND, EXTRA LARGE, 6 PER
1 ALCOHOL PREP PADS 10P
1 O/H PUMP ANTISEPTIC 2 OZ ID F
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 FIRST AID GUIDE ASHI
2 TAPE ADHESIVE 1"X 5 YD PLSTC
10 HAND SANITIZER 0.9G WJ BULK
4 GAUZE CLEAN-WRAP BDGE N/S 2"
4 GAUZE CLEAN-WRAP BDGE N/S 4"
4 BLOODSTOPPER
1 NON-ADHERENT PADS 2"X3" 10'S
1 GZE PADS STERILE 2"X 2" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 COTTON TIPS 100 PER VIAL
1 AYPANAL NON-ASP IND 2/ENV 100
1 CEDAPRIN (IBUPROFEN) 2ENV 100
1 MIRALAC TABS IND PK 2/ENV 100
1 SINUS DECONGESTANT 2/ENV 100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 POCKET INSERT RED #400 KIT 5R
1 TONGUE BLADES SR WRAPPED 6'S
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
4 PR LRG NITRILE GLVES ZIP BAG
2 WATER-JEL BURN DRESSING 4 X 4
1 LBL CONTENTS ANSI 2015 CL B
1 LBL CAB CVR ANSI 2015 CL B
2 TRI BNDG NON WOVEN 40"X40"X56"
2 COLD PACK UNIT 4"X6" BULK
1 RED BIO BAGS 2/BX
4392 Kit Label
SF00004422













