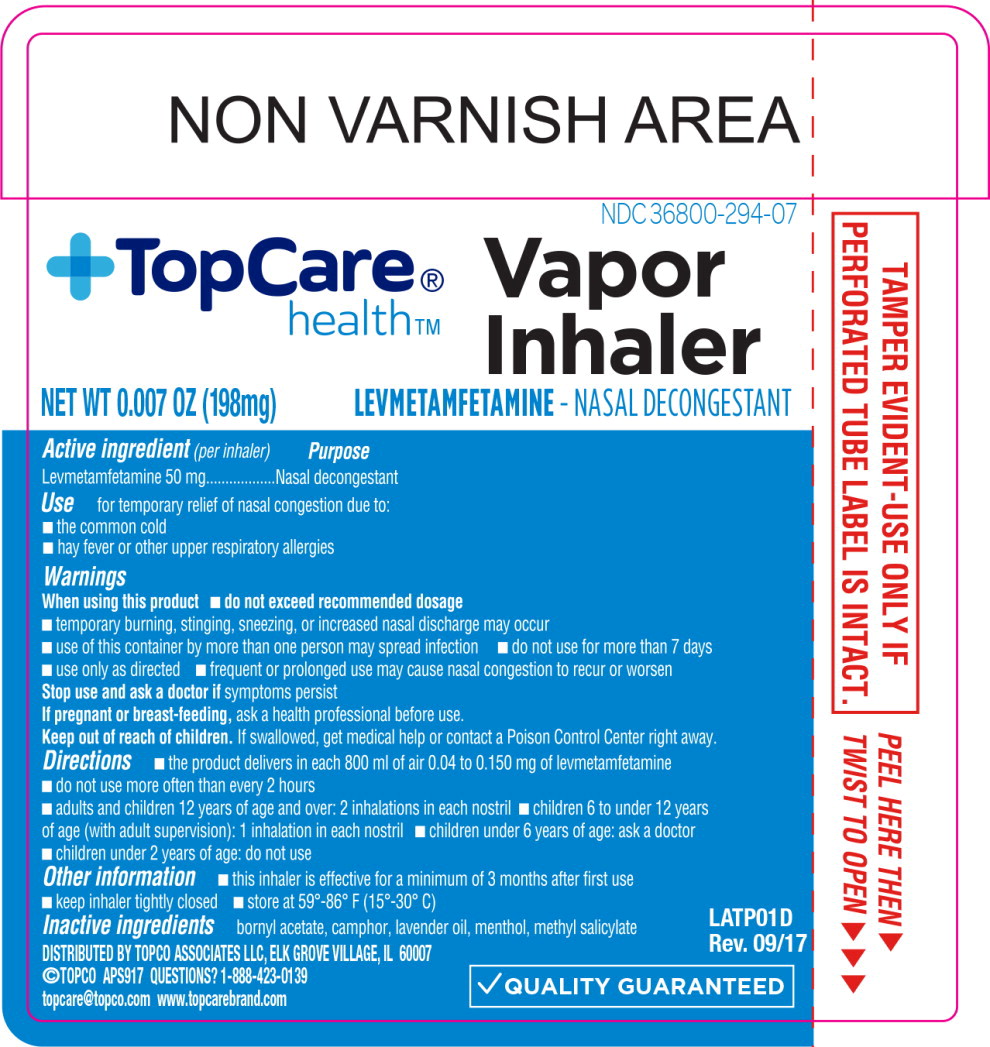
VAPOR INHALER LEVMETAMFETAMINE NASAL DECONGESTANT, TOPCARE HEALTH
- levmetamfetamine inhalant
Topco Associates LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
per inhaler:
Levmetamfetamine 50mg
Purpose
Nasal Decongestant
Use:
For temporary relief of nasal congestion due to
- the common cold
- hay fever or upper respiratory allergies
Warnings
When using this product
-
do not exceed recommended dosage
- temporary burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
- do not use for more than 7 days
- use only as directed
- frequent or prolonged use may cause nasal congestion to recur or worsen
Stop use and ask a doctor if symptoms persist
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- the product delivers in each 800 ml of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
| adults and children 12 years of age and over
| 2 inhalations in each nostril
|
| children 6 to under 12 years of age (with adult supervision)
| 1 inhalation in each nostril
|
| children under 6 years of age
| ask a doctor
|
| children under 2 years of age
| do not use
|
Other information
this inhaler is effective for a minimum of 3 months after first use • keep inhaler tightly closed • store at 59°-86° F (15°-30° C)
Inactive ingredients
bornyl acetate, camphor, lavender oil, menthol, methyl salicylate
Principal Display Panel - 198 mg Blister Pack Label
NDC 36800-294-07
+TopCare® health™
Vapor
Inhaler
OUR PHARMACISTS
RECOMMENDED
LEVMETAMFETAMINE - NASAL DECONGESTANT
Temporary Relief of Nasal
Congestion Due to:
• Colds • Hay Fever
• Upper Respiratory Allergies
NET WT 0.007 OZ (198mg)
Principal Display Panel - 198 mg Inhaler Label
NDC 36800-294-07
+TopCare®
health™
Vapor
Inhaler
LEVMETAMFETAMINE - NASAL DECONGESTANT
NET WT 0.007 OZ (198mg)