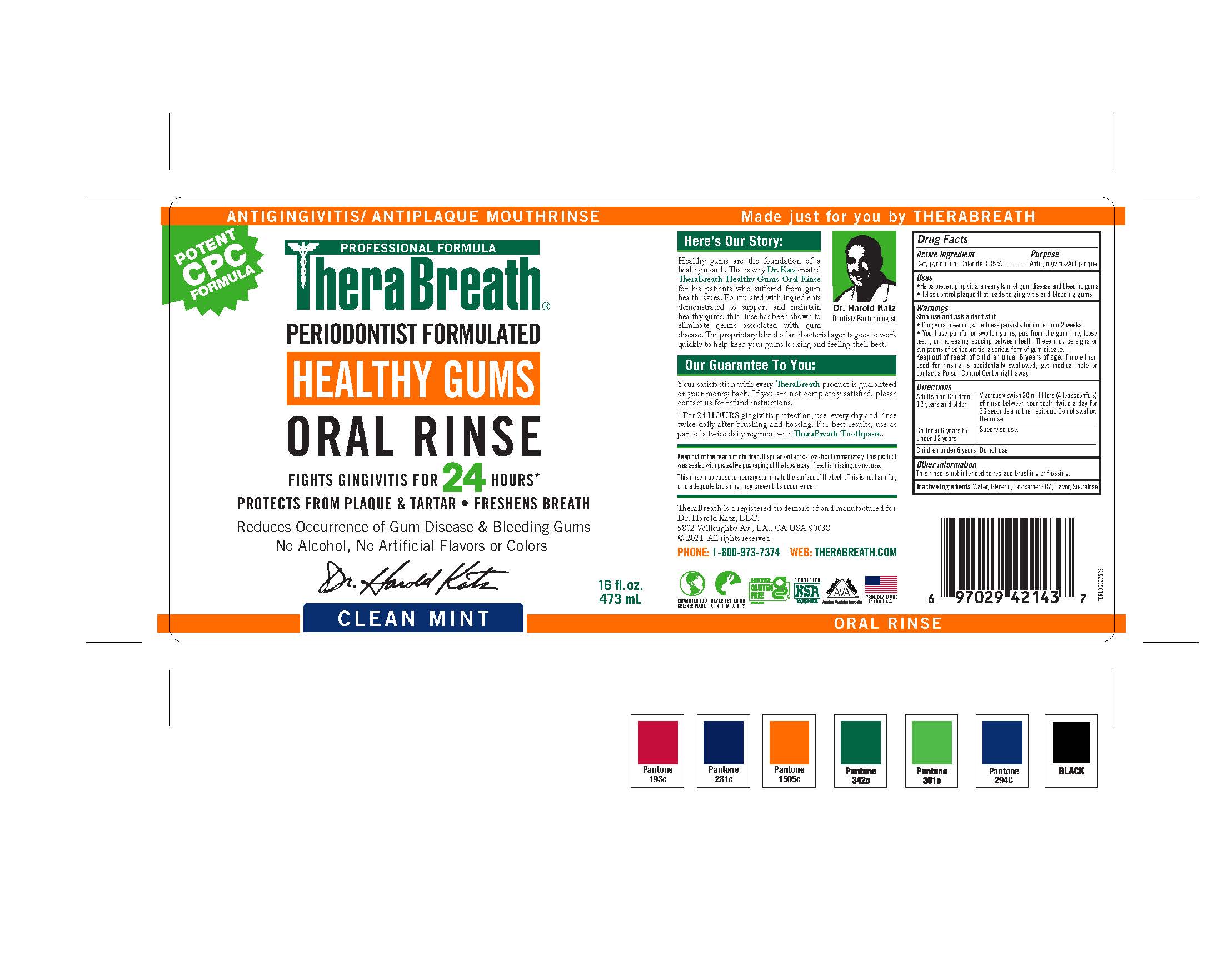
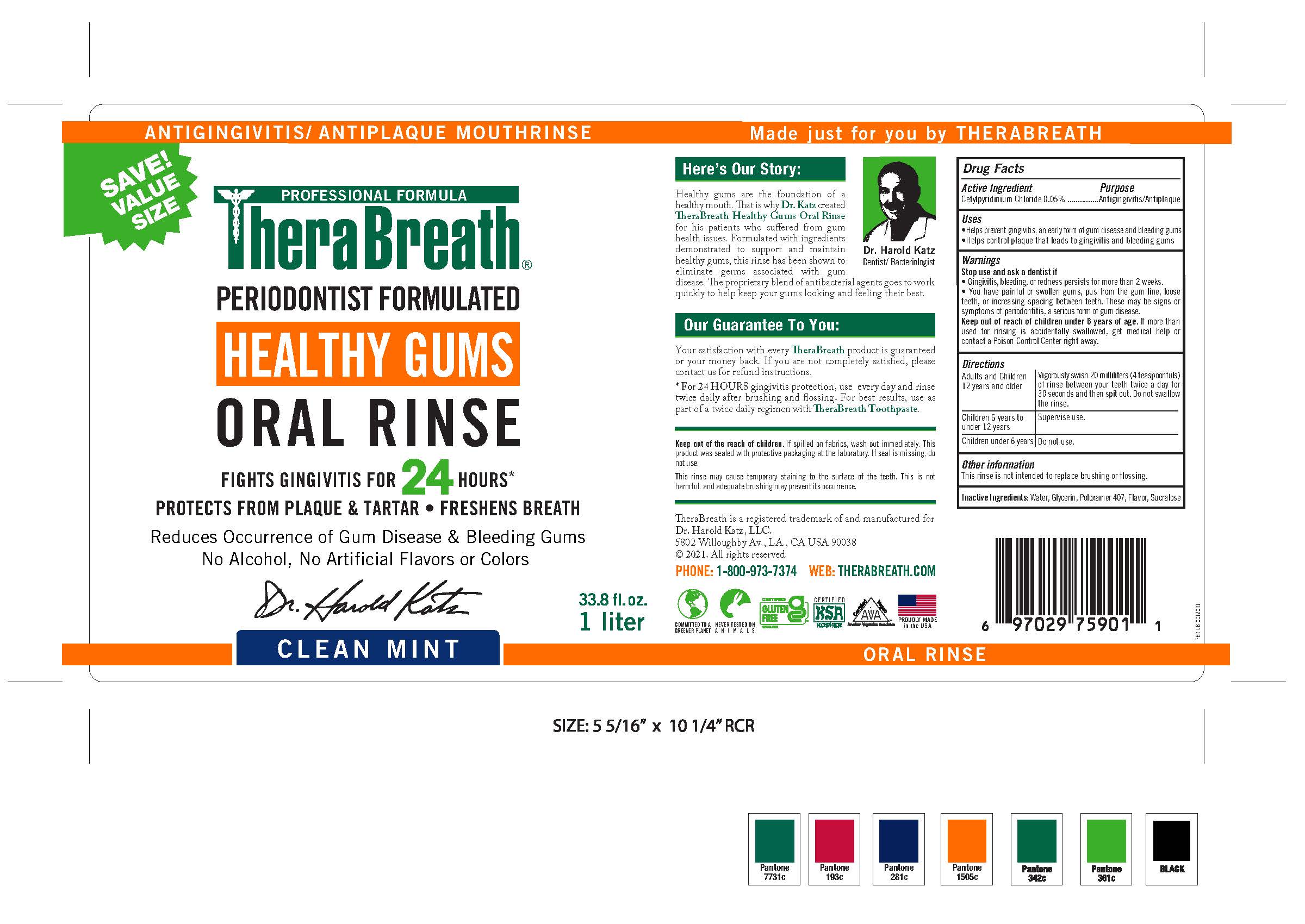
THERABREATH HEALTHY GUMS ORAL RINSE- cetylpyridinium chloride rinse
Dr. Harold Katz, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Cetylpyridinium chloride 0.05%
Purpose
Antigingivitis/Antiplaque
Uses
•Helps prevent gingivitis, an early form of gum disease and bleeding gum
•Helps control plaque that leads to gingivitis and bleeding gums
Warnings
Keep out of reach of children under 6 years of age. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Warnings
Stop Use and Ask a Doctor If
- Gingivitis, bleeding, or redness persists for more than 2 weeks.
- You have painful or swollen gums, pus from the gum line, loose teeth, or increasing spacing between teeth. These may or symptoms of periodontitis, a serious form of gum disease.
Directions
| Adults and Children 12 years and older |
Vigorously swish 20 milliliters (4 teaspoonfuls) of rinse between your teeth
twice a day for 30 seconds and then spit out. Do not swallow the rinse.
|
| Children 6 years to under12 years | supervise use. |
| Children under 6 years | Do not use. |
Other Information
This rinse is not intended to replace brushing or flossing.
Inactive Ingredients
Water, Glycerin, Poloxamer 407, Flavor, Sucralose
TheraBreath Healthy Gums Oral Rinse
72551-247-05
72551-247-01
72551-247-04

Dr. Harold Katz, LLC


