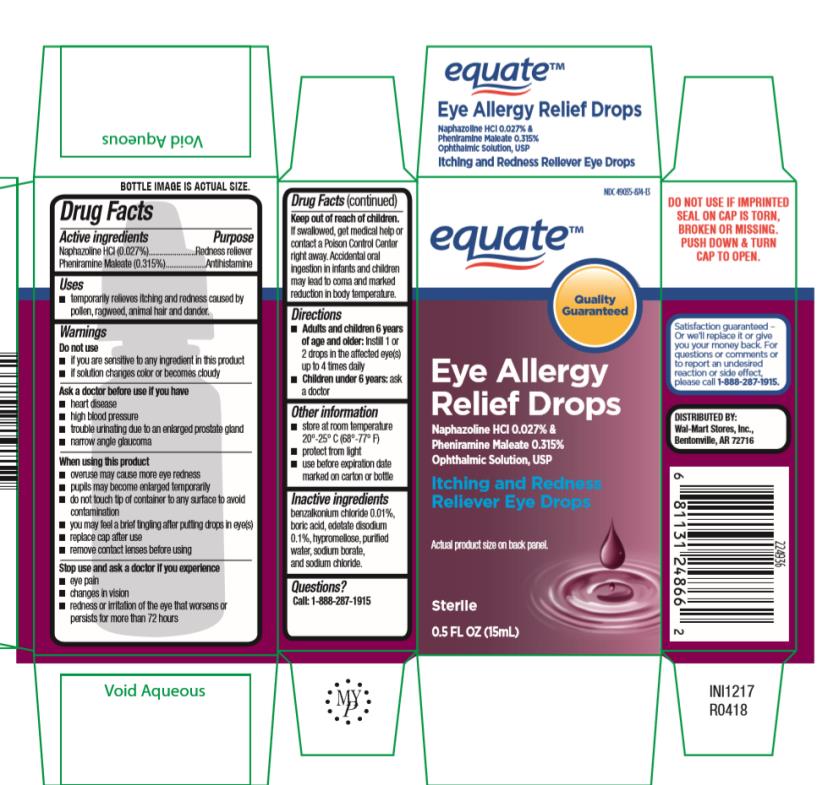
EQUATE EYE ALLERGY RELIEF DROPS- naphazoline hydrochloride, pheniramine maleate solution/ drops
Wal-Mart Stores, Inc.
----------
Walmart
Equate
Eye Allergy Relief Drops 15mL
NDC 49035-874-13
Drug Facts
Active ingredients
Naphazoline HCl (0.027%)
Pheniramine Maleate(0.315%)
Purpose
Redness Reliever
Antihistamine
Uses
- temporarily relieves itching and redness caused by pollen, ragweed, animal hair and dander.
Warnings
Do not use
- if you are sensitive to any ingredient in this product.
- if this product changes color or becomes cloudy.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- trouble urinating due to enlarged prostate gland
- narrow angle glaucoma
When using this product
- overuse may cause more eye redness
- pupils may become enlarged temporarily
- do not touch tip of container to any surface to avoid contamination
- you may feel a brief tingling after putting drops in eye(s)
- replace cap after using
- remove contact lenses before using
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- redness or irritation of the eye that worsens or persists for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. Accidental oral ingestion in infants and children may lead to coma and marked reduction of body temperature.
Directions
-
Adults and children 6 years of age and older: Instill 1 or 2 drops in the affected eye(s) up to 4 times daily
-
Children under 6 years: ask a doctor
Other information
- store at room temperature 20°-25°C (68°-77°F).
- protect from light
- use before expiration date marked on carton or bottle
Inactive ingredients
benzalkonium chloride 0.01%, boric acid, edetate disodium 0.1%, hypromellose, purified water, sodium borate and sodium chloride.
Questions?
Call: 1 888- 287-1915
PRINCIPAL DISPLAY PANEL
NDC 49035-874-13
equate
Eye Allergy
Relief Drops
Itching and Redness
Reliever Eye Drops
Wal-Mart Stores, Inc.
