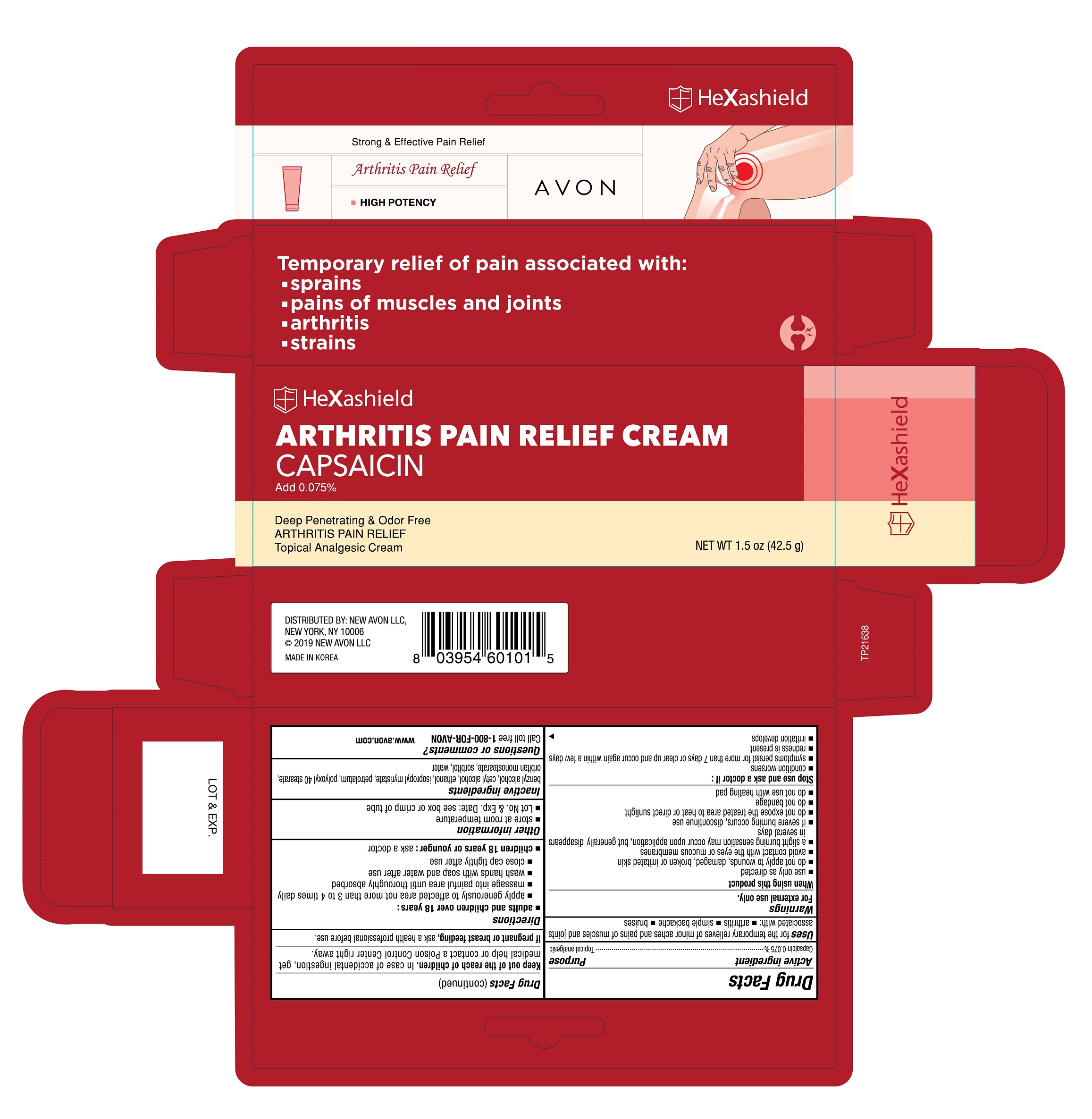
Uses
for temporary relieves of minor aches and pains of muscles and joints associated with:
- arthritis
- simple backache
- bruises
Warnings
For external use only.
When using this product
- use only as directed
- do not apply to wounds, damaged, broken or irritated skin
- avoid contact with the eyes or mucous membranes
- a slight burning sensation may occur upon application, but generally disappears in several days
- if severe burning occurs, discontinue use
- do not expose the treated area to heat or direct sunlight
- do not banage
- do not use with heating pad
Directions
-
adults and children over 18 years :
- apply generously to affected area not more than 3 to 4 times daily
- massage into painful area until thoroughly absorbed
- wash hands with soap and water after use
- close cap tightly after use
- children 18 years or younger : ask a doctor
Inactive Ingredients
benzyl alcohol, cetyl alcohol, ethanol, isopropyl myristate, petrolatum, polyoxyl 40 stearate, orbitan monostearate, sorbitol, water
Principal Display Panel
Strong & Effective Pain Relief
Arthritis Pain Relief
High Potency
AVON
Temporary relief of pain associated with:
- sprains
- pains of muscles and joints
- arthritis
- strains
HeXashield
ARTHRITIS PAIN RELIEF CREAM
CAPSAICIN
Add 0.075%
Deep Penetratng & Odor Free
ARTHRITIS PAIN RELIEF
Topical Analgesic Cream
NET WT 1.5 oz (43.5 g)