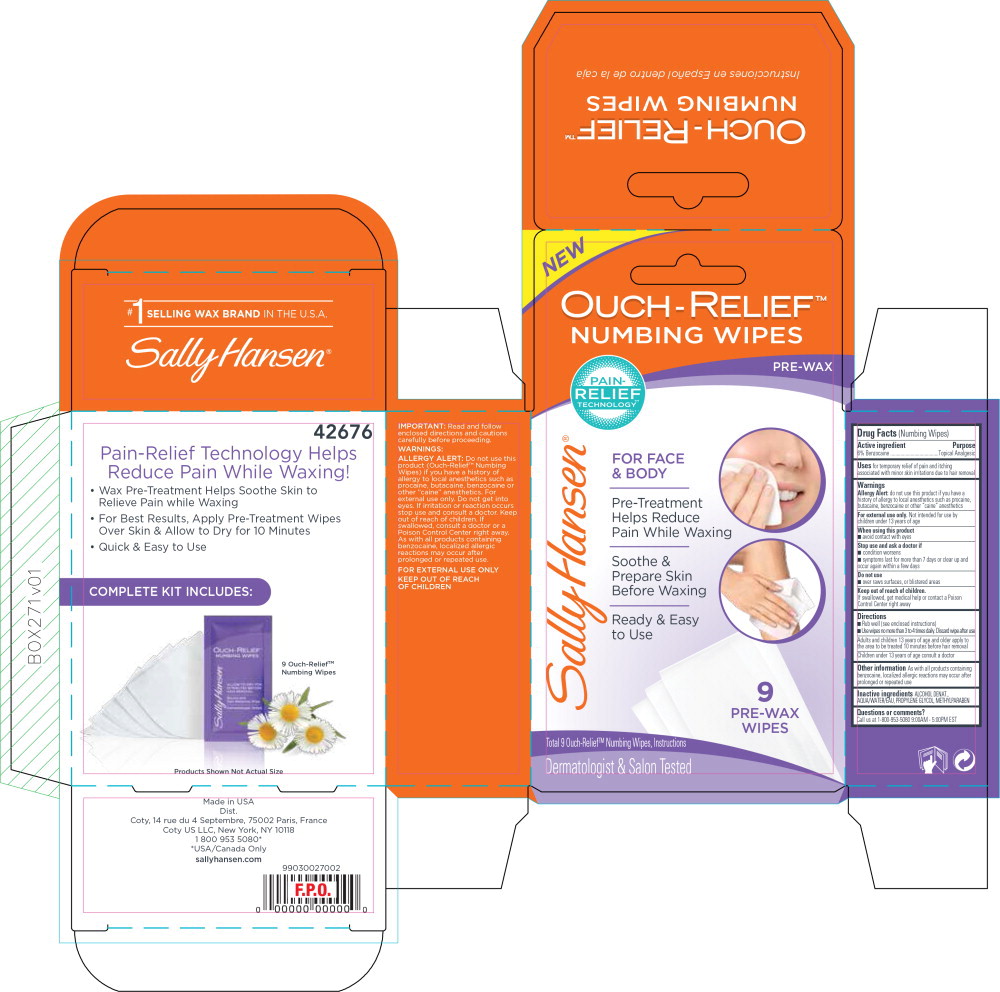
Uses
for temporary relief of pain and itching associated with minor skin irritations due to hair removal
Warnings
Allergy Alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
For external use only. Not intended for use by children under 13 years of age
Directions
- Rub well (see enclosed instructions)
- Use wipes no more than 3 to 4 times daily. Discard wipe after use
Adults and children 13 years of age and older apply to :he area to be treated 10 minutes before hair removal Children under 13 years of age consult a doctor
Other information
As with all products containing benzocaine, localized allergic reactions may occur after prolonged or repeated use
Principal Display Panel - Carton Label
OUCH-RELIEF™
NUMBING WIPES
PRE-WAX
PAIN-
RELIEF
TECHNOLOGY
FOR FACE
& BODY
Pre-Treatment
Help Reduce
Pain While Waxing
Soothe &
Prepare Skin
Before Waxing
Ready & Easy
to Use
9
PRE-WAX
WIPES
Total 9 Ouch-Relief™ Numbing Wipes, Instructions
Dermatologist & Salon Tested