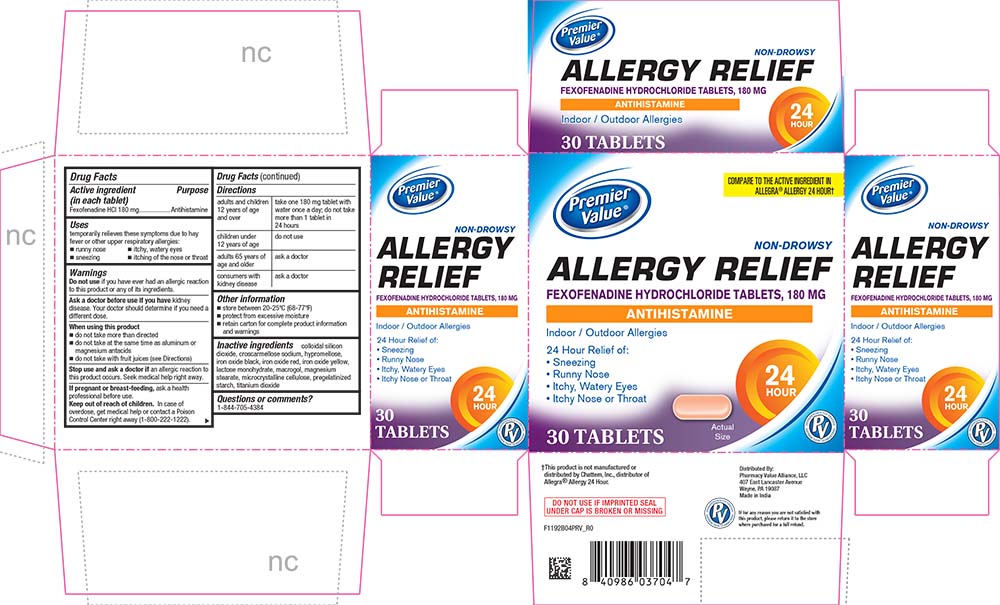
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Directions
|
adults and children 12 years of age and over |
take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours |
|
children under 12 years of age |
do not use |
|
adults 65 years of age and older |
ask a doctor |
|
consumers with kidney disease |
ask a doctor |
Other information
- store between 20-25°C (68-77°F)
- protect from excessive moisture
- retain carton for complete product information and warnings
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, lactose monohydrate, macrogol, magnesium stearate, microcrystalline cellulose, pregelatinized starch, titanium dioxide
PRINCIPAL DISPLAY PANEL
Premier Value®
COMPARE TO THE ACTIVE INGREDIENT IN ALLEGRA® ALLERGY 24 HOUR†
NON-DROWSY
ALLERGY RELIEF
FEXOFENADINE HYDROCHLORIDE TABLETS, 180 MG
ANTIHISTAMINE
Indoor / Outdoor Allergies
24 Hour Relief of:
• Sneezing
• Runny Nose
• Itchy, Watery Eyes
• Itchy Nose or Throat
30 TABLETS
Actual Size