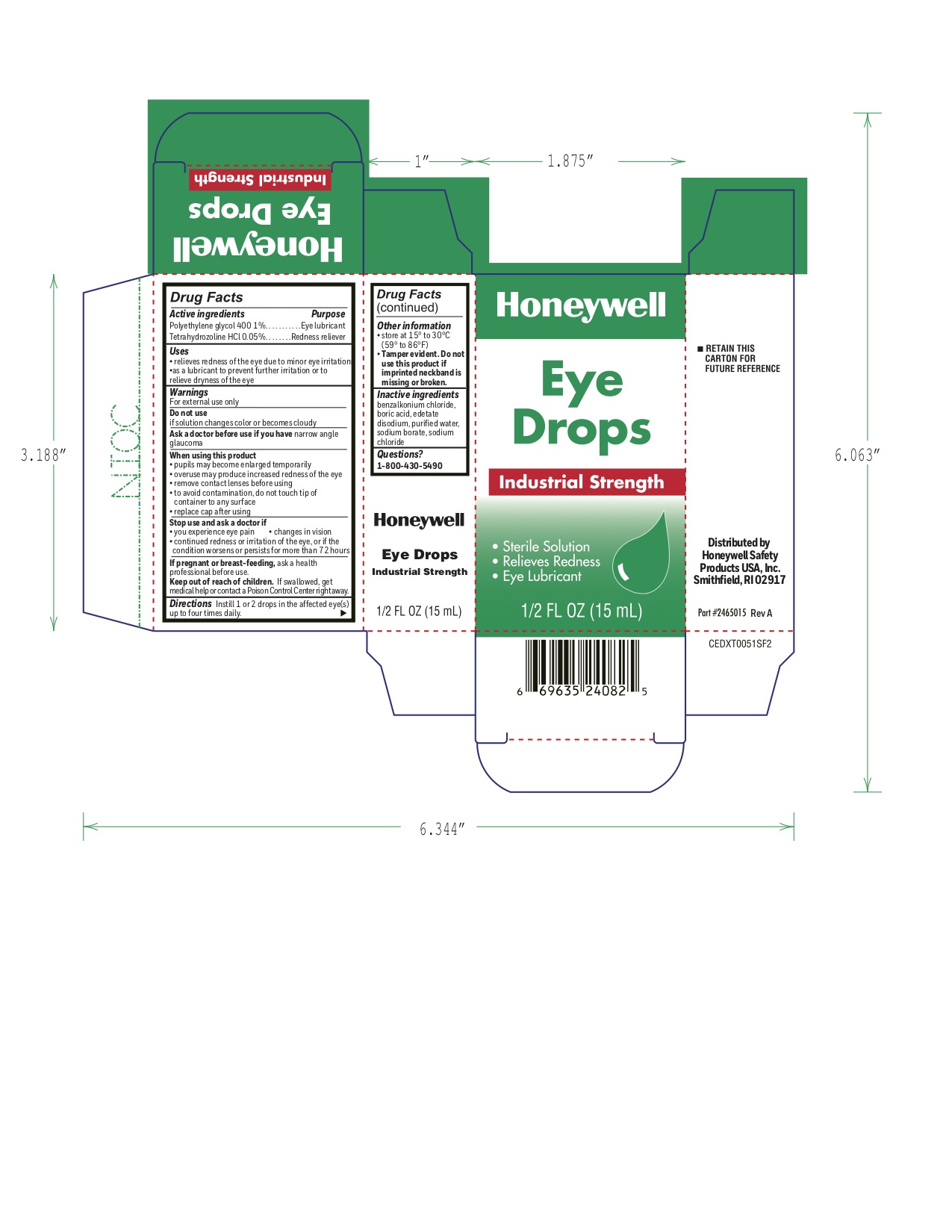
Industrial Strength Eye Drops
Active ingredients
Polyethylene glycol 400 1%
Tetrahydrozoline HCl 0.05%
Industrial Strength Eye Drops
Uses
- relieves redness of the eye due to minor eye irritations
- as a lubricant to prevent further irritation or to relieve dryness of the eye
Industrial Strength Eye drops
Warnings
For external use only
When using this product
- pupils may become enlarged temporarily
- overuse may produce increased redness of the eye
- remove contact lenses before using
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using
Industrial Strength Eye Drops
Directions
Instill 1 or 2 drops in the affected eye(s) up to four times daily.
Industrial Strength Eye Drops
Other information
store at 15° to 30°C (59° to 86°F)
Tamper evident. Do not use this product if imprinted neckband is missing or broken.