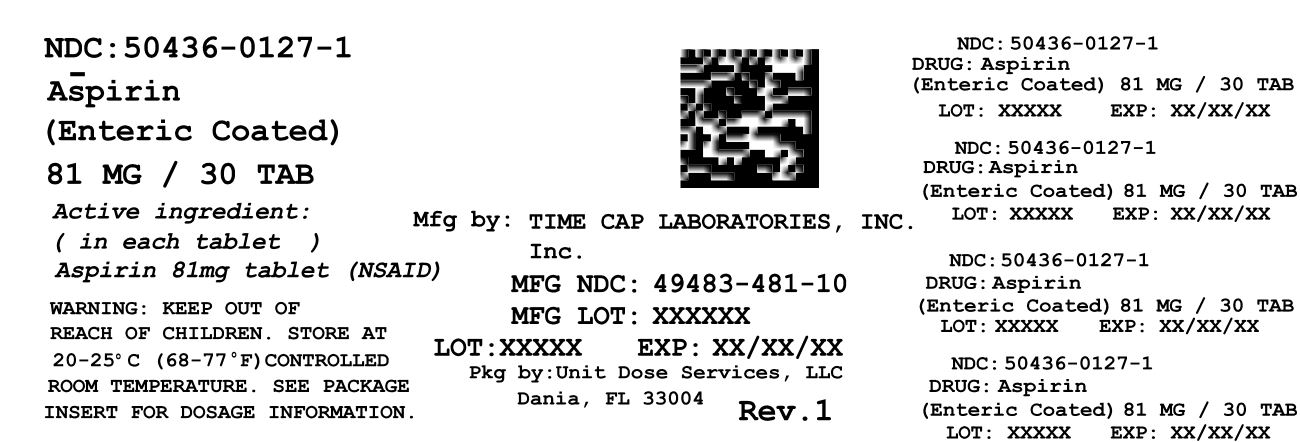
ASPIRIN ENTERIC COATED- aspirin tablet, coated
Unit Dose Services
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ASPIRIN ENTERIC COATED
| ASPIRIN ENTERIC COATED
aspirin tablet, coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Unit Dose Services (831995316) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Unit Dose Services | 831995316 | REPACK(50436-0127) , RELABEL(50436-0127) | |
Revised: 1/2019
Document Id: 8a12f07d-f089-48f9-8a3d-e597a452ebd4
Set id: 8ebcbc13-0323-454a-9834-b6ea326d4e46
Version: 9
Effective Time: 20190117
Unit Dose Services