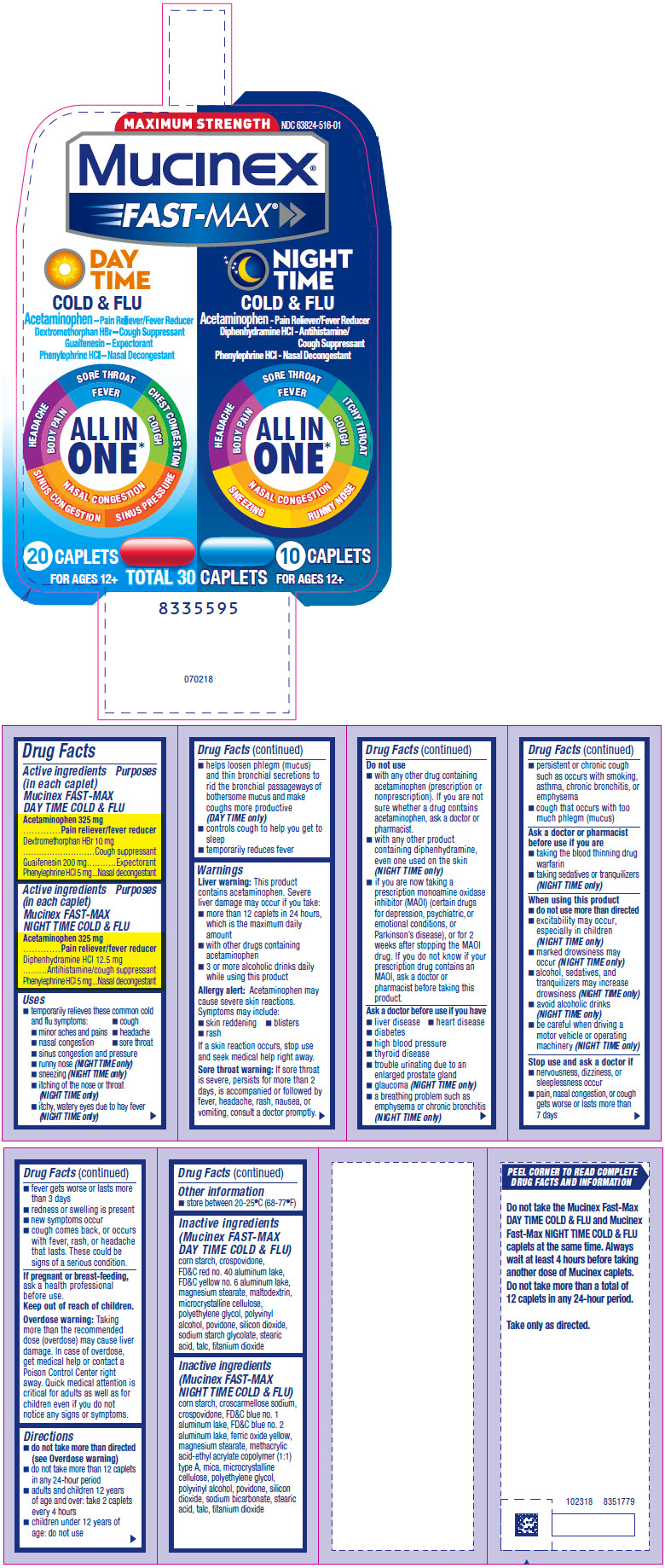
Uses
- temporarily relieves these common cold and flu symptoms:
- cough
- minor aches and pains
- headache
- nasal congestion
- sore throat
- sinus congestion and pressure
- runny nose (NIGHT TIME only)
- sneezing (NIGHT TIME only)
- itching of the nose or throat (NIGHT TIME only)
- itchy, watery eyes due to hay fever (NIGHT TIME only)
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive (DAY TIME only)
- controls cough to help you get to sleep
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on the skin (NIGHT TIME only)
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- trouble urinating due to an enlarged prostate gland
- glaucoma (NIGHT TIME only)
- a breathing problem such as emphysema or chronic bronchitis (NIGHT TIME only)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers (NIGHT TIME only)
When using this product
- do not use more than directed
- excitability may occur, especially in children (NIGHT TIME only)
- marked drowsiness may occur (NIGHT TIME only)
- alcohol, sedatives, and tranquilizers may increase drowsiness (NIGHT TIME only)
- avoid alcoholic drinks (NIGHT TIME only)
- be careful when driving a motor vehicle or operating machinery (NIGHT TIME only)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back, or occurs with fever, rash, or headache that lasts. These could be signs of a serious condition.
Directions
- do not take more than directed (see Overdose warning)
- do not take more than 12 caplets in any 24-hour period
- adults and children 12 years of age and over: take 2 caplets every 4 hours
- children under 12 years of age: do not use
Inactive ingredients (Mucinex FAST-MAX DAY TIME COLD & FLU)
corn starch, crospovidone, FD&C red no. 40 aluminum lake, FD&C yellow no. 6 aluminum lake, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide
Inactive ingredients (Mucinex FAST-MAX NIGHT TIME COLD & FLU)
corn starch, croscarmellose sodium, crospovidone, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, ferric oxide yellow, magnesium stearate, methacrylic acid-ethyl acrylate copolymer (1:1) type A, mica, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium bicarbonate, stearic acid, talc, titanium dioxide
PRINCIPAL DISPLAY PANEL - Kit Carton
MAXIMUM STRENGTH
NDC 63824-516-01
Mucinex®
FAST-MAX
®
DAY
TIME
COLD & FLU
Acetaminophen – Pain Reliever/Fever Reducer
Dextromethorphan HBr – Cough Suppressant
Guaifenesin – Expectorant
Phenylephrine HCl – Nasal Decongestant
HEADACHE
BODY PAIN
SORE THROAT
FEVER
CHEST CONGESTION
COUGH
NASAL CONGESTION
SINUS CONGESTION SINUS PRESSURE
ALL IN
ONE*
20 CAPLETS
FOR AGES 12+
NIGHT
TIME
COLD & FLU
Acetaminophen - Pain Reliever/Fever Reducer
Diphenhydramine HCl - Antihistamine/
Cough Suppressant
Phenylephrine HCl - Nasal Decongestant
HEADACHE
BODY PAIN
SORE THROAT
FEVER
ITCHY THROAT
COUGH
NASAL CONGESTION
SNEEZING RUNNY NOSE
ALL IN
ONE*
10 CAPLETS
FOR AGES 12+
TOTAL 30 CAPLETS