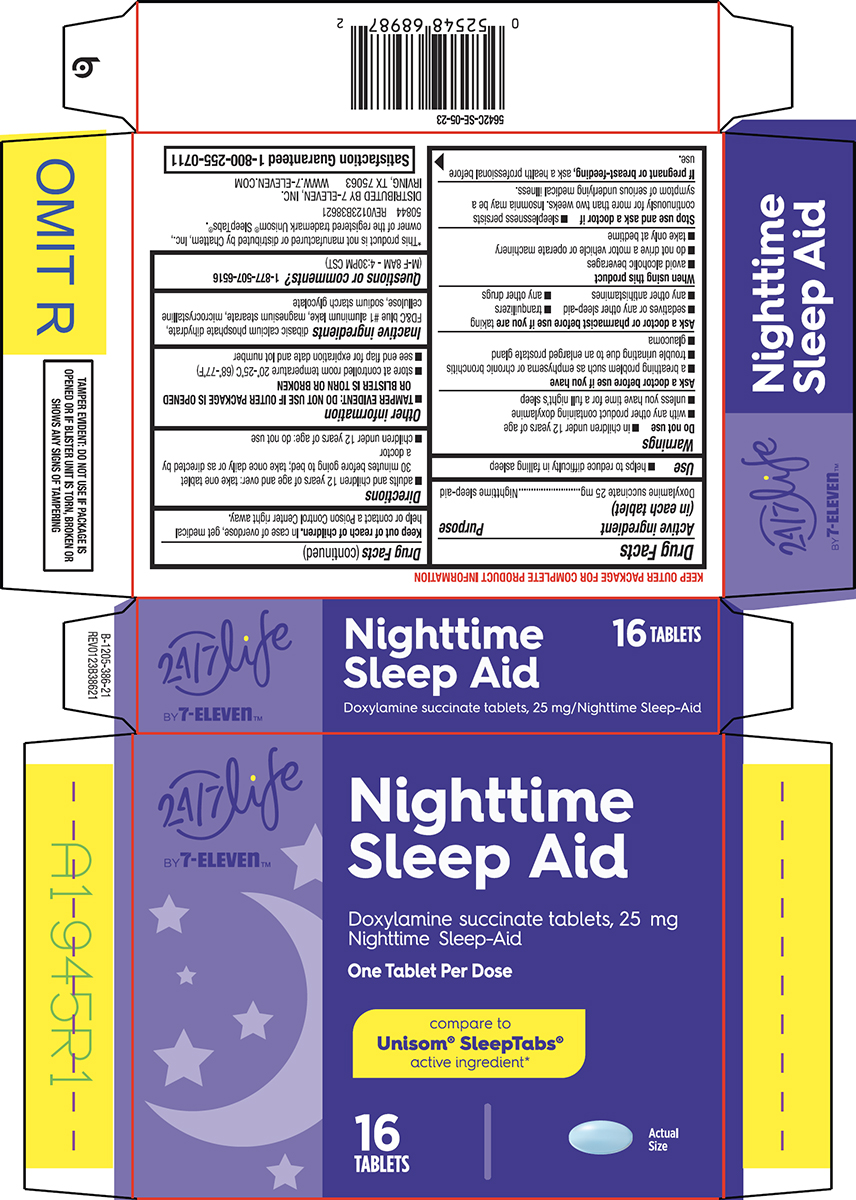
Warnings
Do not use
- in children under 12 years of age
- with any other product containing doxylamine
- unless you have time for a full night’s sleep
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
taking
- sedatives or any other sleep-aid
- tranquilizers
- any other antihistamines
- any other drugs
When using this product
- avoid alcoholic beverages
- do not drive a motor vehicle or operate machinery
- take only at bedtime
Directions
- adults and children 12 years of age and over: take one tablet 30 minutes before going to bed; take once daily or as directed by a doctor
- children under 12 years of age: do not use
Other information
-
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at controlled room temperature 20°-25°C (68°-77°F)
- see end flap for expiration date and lot number
Inactive ingredients
dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
Principal display panel
24/7 Life
BY 7-Eleven™
Nighttime
Sleep Aid
Doxylamine succinate tablets, 25 mg
Nighttime Sleep-Aid
One Tablet Per Dose
compare to
Unisom® SleepTabs®
active ingredient*
16
TABLETS
Actual
Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR
SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Chattem, Inc.,
owner of the registered trademark Unisom® SleepTabs®.
50844 REV0123B38621
DISTRIBUTED BY 7-ELEVEN, INC.
IRVING, TX 75063 WWW.7-ELEVEN.COM
Satisfaction Guaranteed 1-800-255-0711
Convenience Valet 44-386