FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Tiopronin tablets are indicated, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation in adults and pediatric patients 9 years of age and older with severe homozygous cystinuria, who are not responsive to these measures alone.
Additional pediatric use information is approved for Mission Pharmacal Company’s Thiola (tiopronin) tablets. However, due to Mission Pharmacal Company’s marketing exclusivity rights, this drug product is not labeled with that information.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Adults: The recommended initial dosage in adult patients is 800 mg/day. In clinical studies, the average dosage was about 1,000 mg/day.
Pediatrics: The recommended initial dosage in pediatric patients 9 years of age and older is 15 mg/kg/day. Avoid dosages greater than 50 mg/kg per day in pediatric patients [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
Additional pediatric use information is approved for Mission Pharmacal Company’s Thiola (tiopronin) tablets. However, due to Mission Pharmacal Company’s marketing exclusivity rights, this drug product is not labeled with that information.
Administer tiopronin tablets in 3 divided doses at the same times each day at least one hour before or 2 hours after meals.
Consider starting tiopronin tablets at a lower dosage in patients with history of severe toxicity to d-penicillamine.
2.2 Monitoring
Measure urinary cystine 1 month after starting tiopronin tablets and every 3 months thereafter. Adjust tiopronin tablets dosage to maintain urinary cystine concentration less than 250 mg/L.
Assess for proteinuria before treatment and every 3 to 6 months during treatment [see Warnings and Precautions (5.1)].
Discontinue tiopronin tablets in patients who develop proteinuria, and monitor urinary protein and renal function. Consider restarting tiopronin tablets treatment at a lower dosage after resolution of proteinuria.
3 DOSAGE FORMS AND STRENGTHS
Tablets for oral use:
100 mg tablets: White to off-white round shaped, sugar coated tablets, imprinted with W on one side in black ink.
4 CONTRAINDICATIONS
Tiopronin is contraindicated in patients with hypersensitivity to tiopronin or any other components of tiopronin tablets [see Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Proteinuria
Proteinuria, including nephrotic syndrome, and membranous nephropathy, have been reported with tiopronin use. Pediatric patients receiving greater than 50 mg/kg of tiopronin per day may be at increased risk for proteinuria [see Dosage and Administration (2.2), Adverse Reactions (6.1, 6.2), Use in Specific Populations (8.4)]. Monitor patients for the development of proteinuria and discontinue therapy in patients who develop proteinuria [see Dosage and Administration (2.2)].
5.2 Hypersensitivity Reactions
Hypersensitivity reactions (drug fever, rash, fever, arthralgia and lymphadenopathy) have been reported [see Contraindications (4)].
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Proteinuria [see Warnings and Precautions (5.1)]
- Hypersensitivity [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of the drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions occurring at an incidence of ≥5% in an uncontrolled trial in 66 patients with cystinuria age 9 to 68 years are shown in the table below. Patients in group 1 had previously been treated with d-penicillamine; those in group 2 had not. Of those patients who had stopped taking d-penicillamine due to toxicity (34 out of 49 patients in group 1), 22 were able to continue treatment with tiopronin. In those without prior history of d-penicillamine treatment, 6% developed reactions of sufficient severity to require tiopronin withdrawal.
Table 1 presents adverse reactions ≥5% in either treatment group occurring in this trial.
|
System Organ Class |
Adverse Reaction |
Group 1 Previously treated with d-penicillamine (N=49) |
Group 2 Naïve to d-penicillamine (N=17) |
|
Blood and Lymphatic System Disorders |
anemia |
1 (2%) |
1 (6%) |
|
Gastrointestinal Disorders |
nausea |
12 (25%) |
2 (12%) |
|
emesis |
5 (10%) |
– |
|
|
diarrhea/soft stools |
9 (18%) |
1 (6%) |
|
|
abdominal pain |
– |
1 (6%) |
|
|
oral ulcers |
6 (12%) |
3 (18%) |
|
|
General Disorders and Administration Site Conditions |
fever |
4 (8%) |
– |
|
weakness |
2 (4%) |
2 (12%) |
|
|
fatigue |
7 (14%) |
– |
|
|
peripheral (edema) |
3 (6%) |
1 (6%) |
|
|
chest pain |
– |
1 (6%) |
|
|
Metabolism and Nutrition Disorders |
anorexia |
4 (8%) |
– |
|
Musculoskeletal and Connective Tissue Disorders |
arthralgia |
– |
2 (12%) |
|
Renal and Urinary Disorders |
proteinuria |
5 (10%) |
1 (6%) |
|
impotence |
– |
1 (6%) |
|
|
Respiratory, Thoracic and Mediastinal Disorders |
cough |
– |
1 (6%) |
|
Skin and Subcutaneous Tissue Disorders |
rash |
7 (14%) |
2 (12%) |
|
ecchymosis |
3 (6%) |
– |
|
|
pruritus |
2 (4%) |
1 (6%) |
|
|
urticaria |
4 (8%) |
– |
|
|
skin wrinkling |
3 (6%) |
1 (6%) |
Taste Disturbance
A reduction in taste perception may develop. It is believed to be the result of chelation of trace metals by tiopronin. Hypogeusia is often self-limited.
6.2 Postmarketing Experience
Adverse reactions have been reported from the literature, as well as during postapproval use of tiopronin. Because the postapproval reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to tiopronin exposure.
Adverse reactions reported during the postmarketing use of tiopronin are listed by body system in Table 2.
|
System Organ Class |
Preferred Term |
|
Cardiac Disorders |
congestive heart failure |
|
Ear and Labyrinth Disorder |
vertigo |
|
Gastrointestinal Disorders |
abdominal discomfort; abdominal distension; abdominal pain; chapped lips; diarrhea; dry mouth; dyspepsia; eructation; flatulence; gastrointestinal disorder; gastroesophageal reflux disease; nausea; vomiting; jaundice; liver transaminitis |
|
General Disorders and Administration Site Conditions |
asthenia; chest pain; fatigue; malaise; pain; peripheral swelling; pyrexia; swelling |
|
Investigations |
glomerular filtration rate decreased; weight increased |
|
Metabolism and Nutrition Disorders |
decreased appetite; dehydration; hypophagia |
|
Musculoskeletal and Connective Tissue Disorders |
arthralgia; back pain; flank pain; joint swelling; limb discomfort; musculoskeletal discomfort; myalgia; neck pain; pain in extremity |
|
Nervous System Disorders |
ageusia; burning sensation; dizziness; dysgeusia; headache; hypoesthesia |
|
Renal and Urinary Disorders |
nephrotic syndrome; proteinuria; renal failure |
|
Skin and Subcutaneous Tissue Disorders |
dry skin; hyperhidrosis; pemphigus foliaceus; pruritus; rash; rash pruritic; skin irritation; skin texture abnormal; skin wrinkling; urticaria |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available published case report data with tiopronin have not identified a drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. Renal stones in pregnancy may result in adverse pregnancy outcomes (see Clinical Considerations). In animal reproduction studies, there were no adverse developmental outcomes with oral administration of tiopronin to pregnant mice and rats during organogenesis at doses up to 2 times a 2 grams/day human dose (based on mg/m2). The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Renal stones in pregnancy may increase the risk of adverse pregnancy outcomes, such as preterm birth and low birth weight.
Data
Animal Data
No findings of fetal malformations could be attributed to the drug in reproduction studies in mice and rats at doses up to 2 times the highest recommended human dose of 2 grams/day (based on mg/m2).
8.2 Lactation
Risk Summary
There are no data on the presence of tiopronin in either human or animal milk or on the effects of the breastfed child. A published study suggests that tiopronin may suppress milk production. Because of the potential for serious adverse reactions, including nephrotic syndrome, advise patients that breastfeeding is not recommended during treatment with tiopronin.
8.4 Pediatric Use
Tiopronin is indicated in pediatric patients 9 years of age and older with severe homozygous cystinuria, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation who are not responsive to these measures alone. This indication is based on safety and efficacy data from a trial in patients 9 years to 68 years of age and clinical experience. Proteinuria, including nephrotic syndrome, has been reported in pediatric patients. Pediatric patients receiving greater than 50 mg/kg tiopronin per day may be at greater risk [see Dosage and Administration (2.1, 2.2), Warnings and Precautions (5.1), Adverse Reactions (6.1)].
Tiopronin tablets are not approved for use in pediatric patients weighing less than 20 kg or in pediatric patients unable to swallow tablets [see Dosage and Administration (2.1)].
Additional pediatric use information is approved for Mission Pharmacal Company’s Thiola (tiopronin) tablets. However, due to Mission Pharmacal Company’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
11 DESCRIPTION
Tiopronin immediate-release tablets are a reducing and cystine-binding thiol drug (CBTD) for oral use. Tiopronin is N-(2-Mercaptopropionyl) glycine and has the following structure:
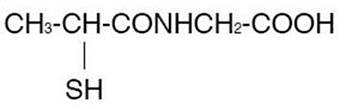
Tiopronin has the empirical formula C5H9NO3S and a molecular weight of 163.20. In this drug product tiopronin exists as a dl racemic mixture.
Tiopronin is a white to off-white crystalline powder, which is freely soluble in water.
Each tiopronin tablet contains 100 mg of tiopronin. The inactive ingredients in tiopronin tablets include colloidal silicon dioxide, corn starch, ethylcellulose, hydroxypropyl cellulose, lactose monohydrate, low substituted hydroxypropyl cellulose, magnesium stearate, silicified microcrystalline cellulose, and stearic acid. The tablets contain a coating that consists of glyceryl monocaprylocaprate, glyceryl monostearate, hypromellose 2910, medium chain triglycerides, polyvinyl alcohol-part hydrolyzed, polyethylene glycol 3350, sodium lauryl sulfate, sucrose, and talc. In addition, the imprinting ink contains ammonium hydroxide, black iron oxide, propylene glycol, and shellac.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The goal of therapy is to reduce urinary cystine concentration below its solubility limit. Tiopronin is an active reducing agent which undergoes thiol-disulfide exchange with cystine to form a mixed disulfide of tiopronin-cysteine. From this reaction, a water-soluble mixed disulfide is formed and the amount of sparingly soluble cystine is reduced.
12.2 Pharmacodynamics
The decrement in urinary cystine produced by tiopronin is generally proportional to the dose. A reduction in urinary cystine of 250 to 350 mg/day at tiopronin dosage of 1 g/day, and a decline of approximately 500 mg/day at a dosage of 2 g/day, might be expected. Tiopronin has a rapid onset and offset of action, showing a fall in cystine excretion on the first day of administration and a rise on the first day of drug withdrawal.
12.3 Pharmacokinetics
Absorption
Tiopronin Tablets
When tiopronin single doses were given to fasted healthy subjects (n=39), the median time to peak plasma level (Tmax) was 1 (range: 0.5 to 2.1) hours.
Elimination
Excretion
When tiopronin is given orally, up to 48% of dose appears in urine during the first 4 hours and up to 78% by 72 hours.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term carcinogenicity studies in animals have not been performed.
Mutagenesis
Tiopronin was not genotoxic in the chromosomal aberration, sister chromatid exchange, and in vivo micronucleus assays.
Impairment of Fertility
High doses of tiopronin in experimental animals have been shown to interfere with maintenance of pregnancy and viability of the fetus. In 2 published male fertility studies in rats, tiopronin at 20 mg/kg/day intramuscular (IM) for 60 days induced reductions in testis, epididymis, vas deferens, and accessory sex glands weights and in the count and motility of cauda epididymal sperm.
16 HOW SUPPLIED/STORAGE AND HANDLING
100 mg: Each white to off-white round shaped, sugar coated tablet, imprinted with W on one side in black ink contains 100 mg of tiopronin. Tablets are available in bottles of 100 (NDC 0093-7909-01).
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Lactation
Advise women that breastfeeding is not recommended during treatment with tiopronin tablets [see Use in Specific Populations (8.2)].
Manufactured in India By:
Watson Pharma Private Limited
Verna, Salcette Goa 403 722 India
Manufactured For:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
Iss. 2/2021
