ESOMEPRAZOLE MAGNESIUM - esomeprazole magnesium capsule, delayed release
Dolgencorp, LLC
----------
Esomeprazole Magnesium Delayed-Release Capsules USP 20 mg*
Drug Facts
Active ingredient (in each capsule)
*Esomeprazole 20 mg
(Each delayed-release capsule corresponds to 21.75 mg esomeprazole magnesium dihydrate USP)
Uses
- treats frequent heartburn (occurs 2 or more days a week)
- not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
Do not use if you have:
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are
- taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
- you need to take more than 1 course of treatment every 4 months
- you get diarrhea
- you develop a rash or joint pain
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
Other information
- read the directions and warnings before use
- keep the carton. It contains important information.
- store at 20-25°C (68-77°F)
- Meets USP dissolution test 2
Inactive ingredients
colloidal silicon dioxide, FD&C blue no.1, gelatin, hydroxypropyl cellulose, hypromellose, magnesium carbonate, magnesium oxide, methacrylic acid copolymer dispersion, mono and di glycerides, polysorbate 80, propylene glycol, shellac, sodium lauryl sulfate, strong ammonia solution, sugar spheres (which contains liquid glucose, starch (maize) and sucrose), talc, titanium dioxide, triethyl citrate and yellow iron oxide.
Questions or comments?
call 1-888-309-9030
(Monday - Friday 8:30 AM to 5:00 PM EST)
DISTRIBUTED BY DOLGENCORP, LLC
100 MISSION RIDGE
GOODLETTSVILLE,
TN 37072 USA
Made in India
Code: TS/DRUGS/22/2009
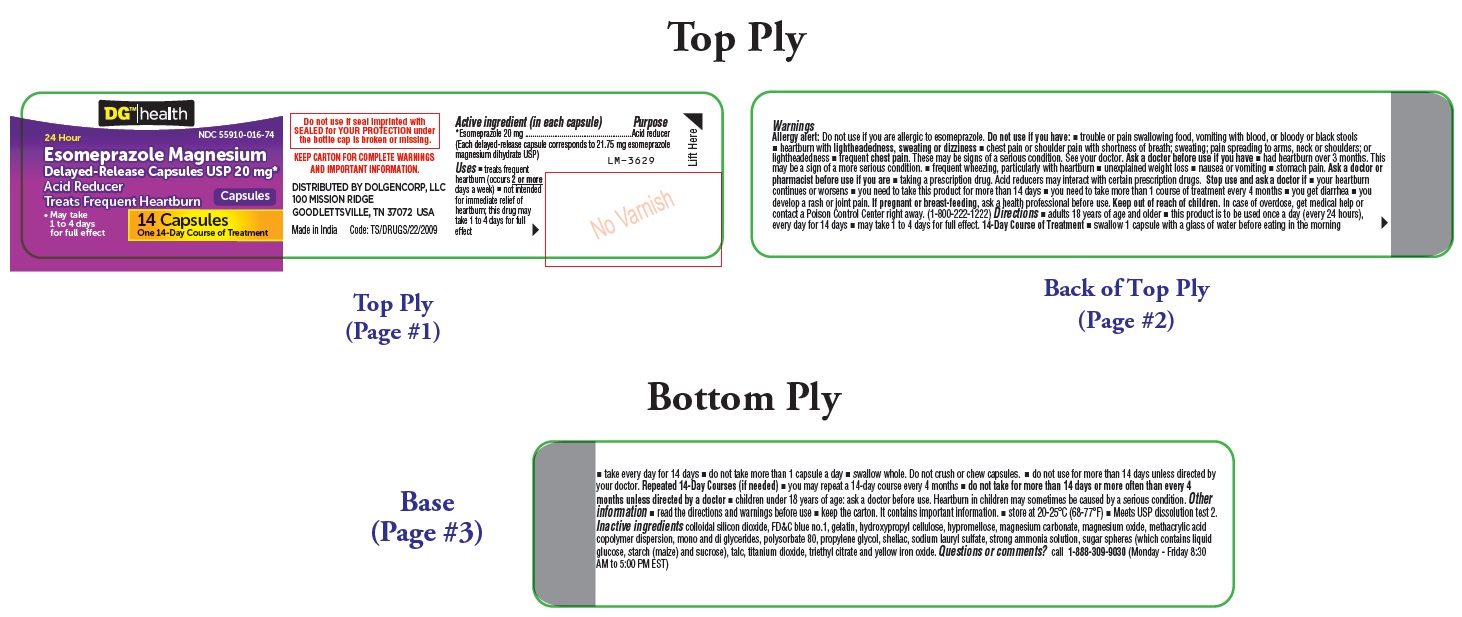
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (14 Capsules Container Label)
DG™ health
NDC 55910-016-74
24 Hour
Esomeprazole Magnesium
Delayed-Release Capsules USP 20 mg*
Acid Reducer
Treats Frequent Heartburn Capsules
- May take 1 to 4 days for full effect
14 Capsules
One 14-Day Course of Treatment
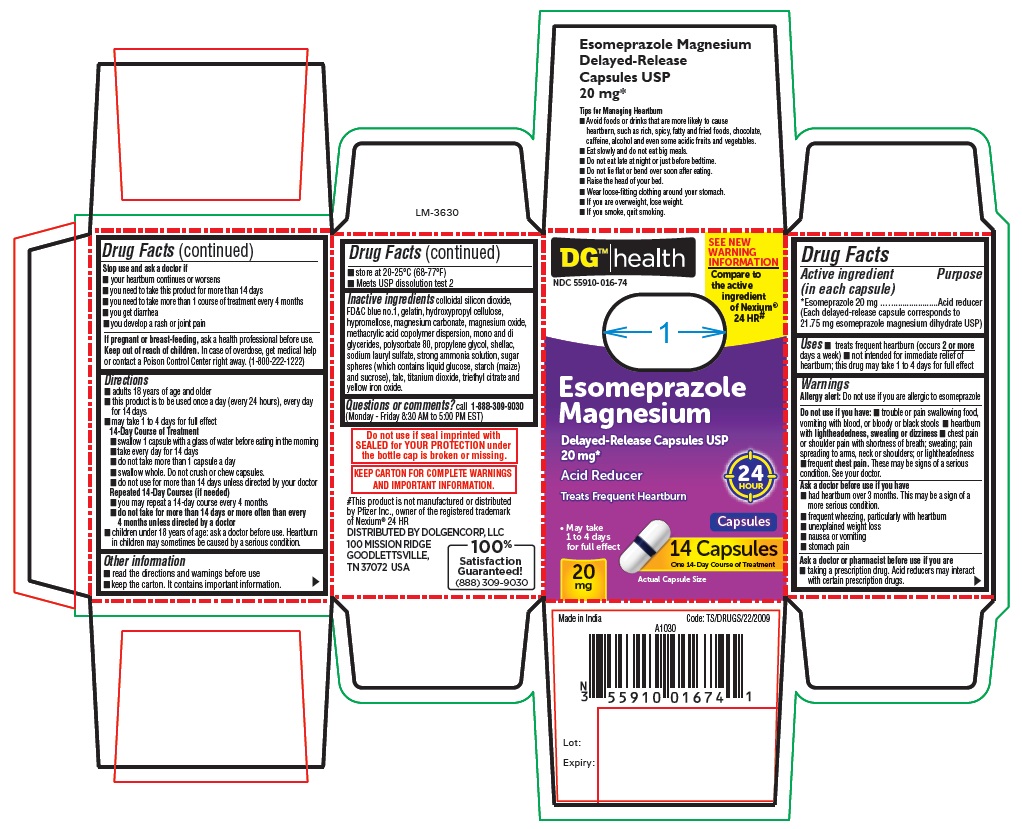
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (14 Capsules Container Carton)
DG™ health
NDC 55910-016-74
SEE NEW WARNING INFORMATION
Compare to the active Ingrdient of Nexium® 24 HR#
Esomeprazole
Magnesium
Delayed-Release Capsules USP
20 mg*
Acid Reducer 24 HOUR
Capsules
Treats Frequent Heartburn
- May take 1 to 4 days for full effect
20 mg
14 CAPSULES
One 14-Day Course of Treatment
Actual Capsule Size
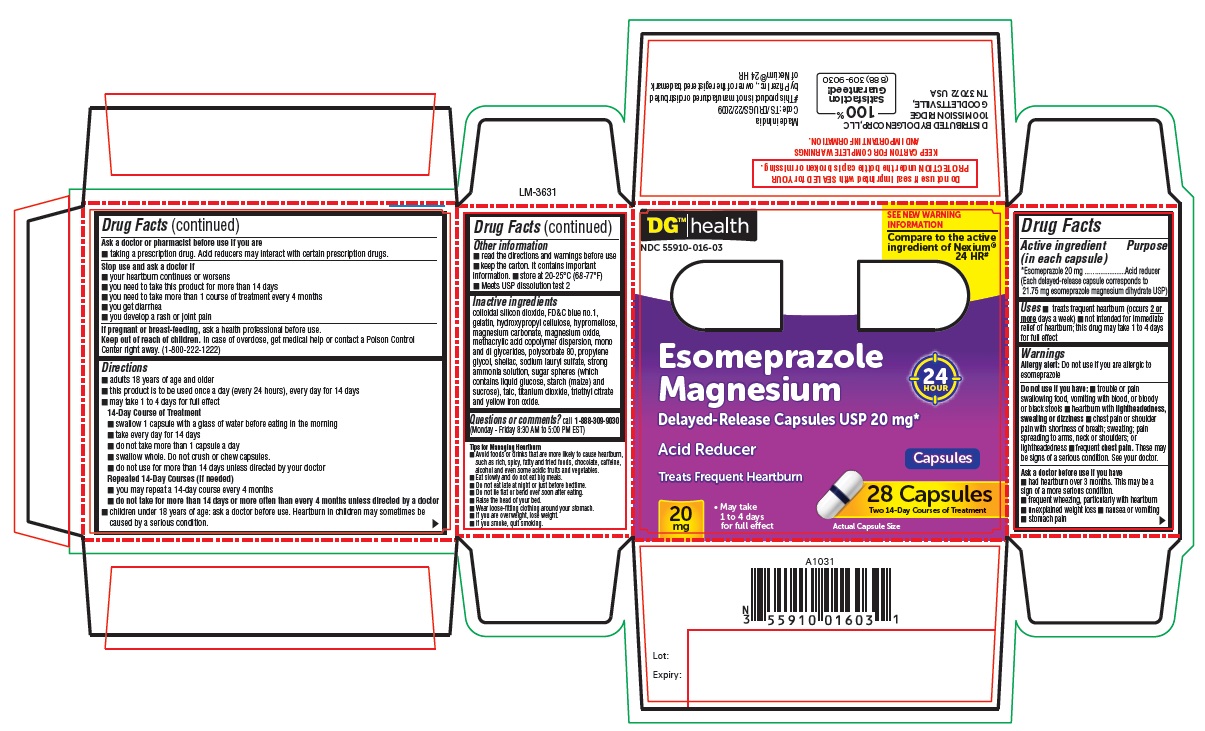
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (28 Capsules Container Carton)
DG™ Health
NDC 55910-016-03
SEE NEW WARNING INFORMATION
Compare to the active ingredient of Nexium® 24 HR#
Esomeprazole
Magnesium
Delayed-Release Capsules USP 20 mg*
Acid Reducer 24 HOUR
Capsules
Treats Frequent Heartburn
20 mg
- May take 1 to 4 days for full effect
28 CAPSULES
Two 14-Day Courses of Treatment
Actual Capsule Size
| ESOMEPRAZOLE MAGNESIUM
esomeprazole magnesium capsule, delayed release |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Dolgencorp, LLC (068331990) |
| Registrant - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(55910-016) , MANUFACTURE(55910-016) | |